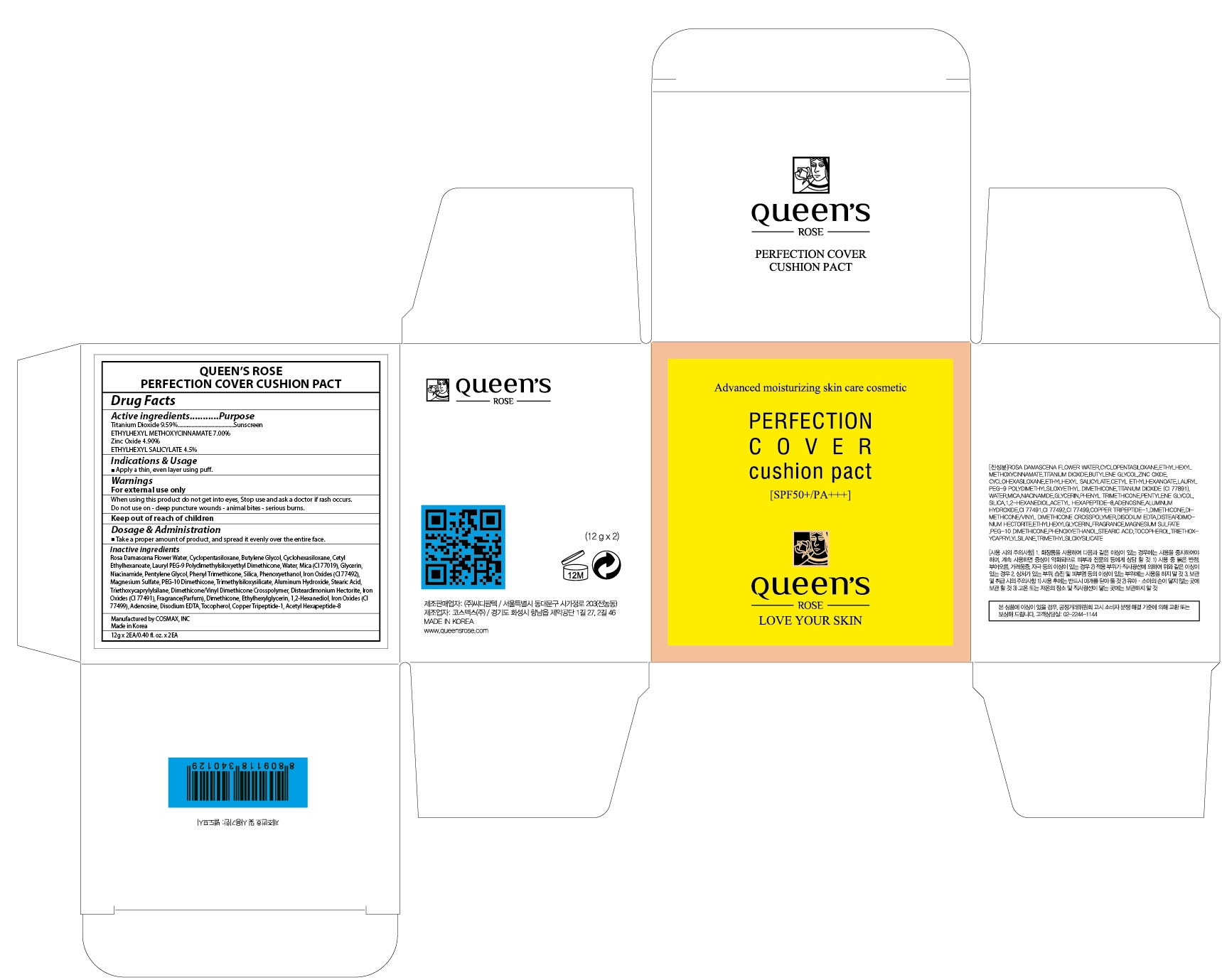
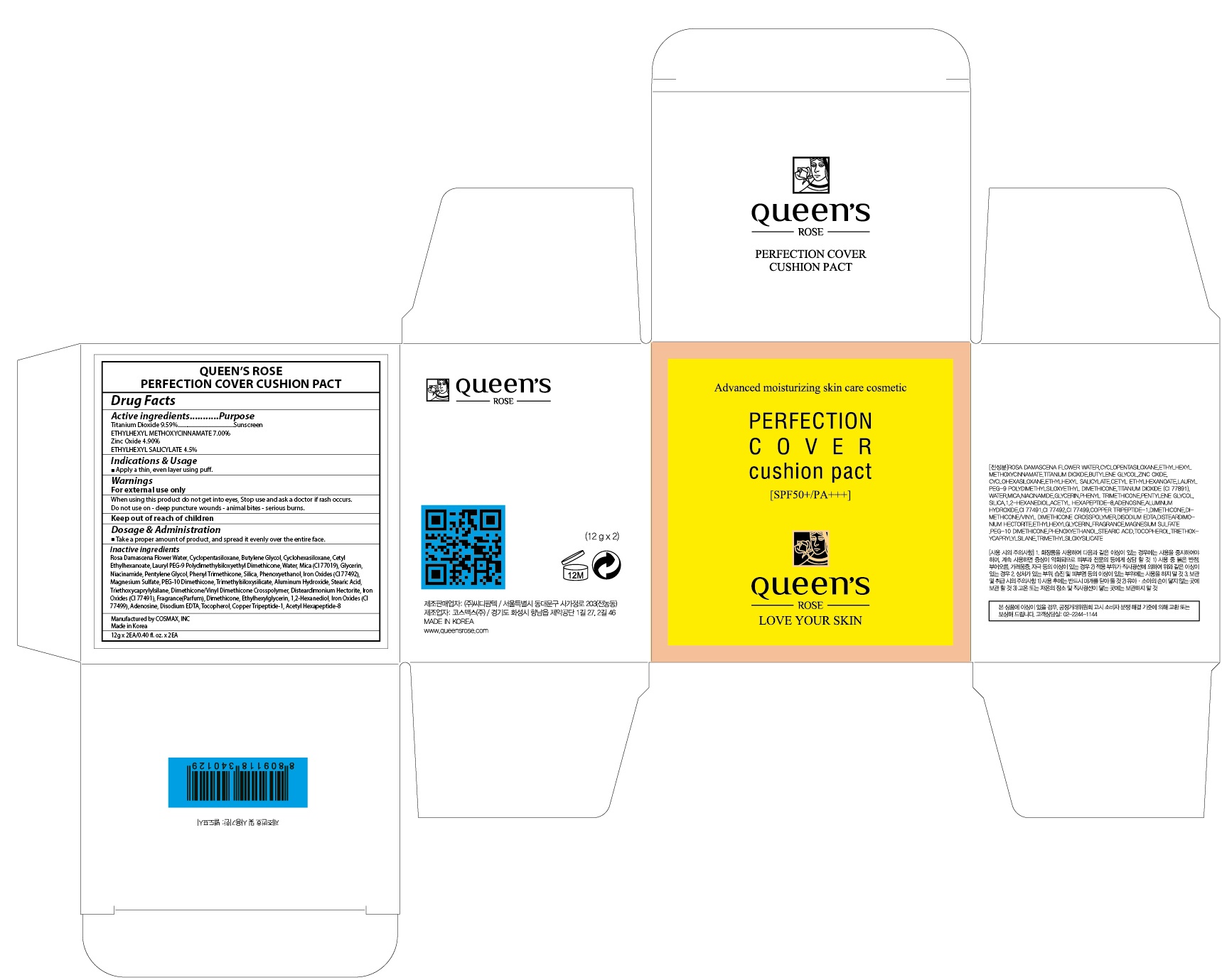
Label: QUEENS ROSE PERFECTION COVER CUSHION PACT- titanium dioxide, octinoxate, zinc oxide, octisalate powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 71252-120-01, 71252-120-02 - Packager: Cdpharmtec Co.,ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 24, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients: Rosa Damascena Flower Water, Cyclopentasiloxane, Butylene Glycol, Cyclohexasiloxane, Cetyl Ethylhexanoate, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Water, Mica (CI 77019), Glycerin, Niacinamide, Pentylene Glycol, Phenyl Trimethicone, Silica, Phenoxyethanol, Iron Oxides (CI 77492), Magnesium Sulfate, PEG-10 Dimethicone, Trimethylsiloxysilicate, Aluminum Hydroxide, Stearic Acid, Triethoxycaprylylsilane, Dimethicone/Vinyl Dimethicone Crosspolymer, Disteardimonium Hectorite, Iron Oxides (CI 77491), Fragrance(Parfum), Dimethicone, Ethylhexylglycerin, 1,2-Hexanediol, Iron Oxides (CI 77499), Adenosine, Disodium EDTA, Tocopherol, Copper Tripeptide-1, Acetyl Hexapeptide-8
- PURPOSE
- WARNINGS
- DESCRIPTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QUEENS ROSE PERFECTION COVER CUSHION PACT
titanium dioxide, octinoxate, zinc oxide, octisalate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71252-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium Dioxide 1.15 g in 12 g Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 0.84 g in 12 g Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.58 g in 12 g Octisalate (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) Octisalate 0.54 g in 12 g Inactive Ingredients Ingredient Name Strength ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) Butylene Glycol (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71252-120-02 2 in 1 CARTON 03/02/2017 1 NDC:71252-120-01 12 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/02/2017 Labeler - Cdpharmtec Co.,ltd (694209262) Registrant - Cdpharmtec Co.,ltd (694209262) Establishment Name Address ID/FEI Business Operations Cdpharmtec Co.,ltd 694209262 relabel(71252-120) Establishment Name Address ID/FEI Business Operations Cosmax, Inc 689049693 manufacture(71252-120)