Label: BACTERIA AND FUNGI AQUARIUM CURE PROGRAM FRESH- acriflavine hydrochloride, methyl orange, ethacridine lactate, methylene blue anhydrous kit
- NDC Code(s): 86048-008-01, 86048-009-01, 86048-014-01
- Packager: Prodibio SAS
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 3, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INSTRUCTIONS
- PRESENTATION
- SPL UNCLASSIFIED SECTION
-
- ACTIVE INGREDIENTS
The vials A and B contain per mL:
• 2.00 mg of 3,6-diamino-10-methylacridinium chloride mixt. with 3,6-diaminoacridine (acriflavine chloride)
• 0.26 mg of 4-(4-dimethylaminophenylazo)-benzenesulfonate sodium salt (methyl orange)
• 4.00 mg of 6,9-diamino-2-ethoxyacridine-DL-lactate monohydrate (ethacridine lactate)
• 2.75 mg of 3,7-bis(dimethylamino)phenazathionium chloride (methylthionine chloride)
The BioDigest Start vial contains nitrifying and denitrifying living bacteria that have not been genetically modified. - - EXCIPIENTS
- - PHARMACEUTICAL FORM & CONTENTS
- - MANUFACTURER
-
USAGE
- THERAPEUTIC INDICATIONS
Product intended only for the treatment of ornamental freshwater fish. Bacteria & Fungi Fresh has a wide spectrum of action and treats a large number of diseases caused by bacteria or fungi. Visible symptoms may be whitish or yellowish secretions, ulcers, skin inflammations, bleeding, scale loss, fin decomposition, bulging eyes, cotton fluff, swollen belly, or unusual oscillatory movements of the fish. Bacteria & Fungi Fresh can be used when the diagnosis of the disease is neither possible nor obvious. -
- DOSAGE AND DIRECTIONS FOR USE
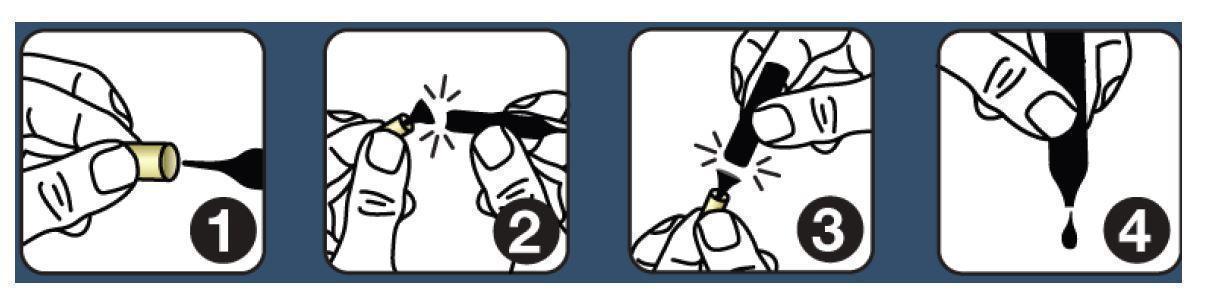
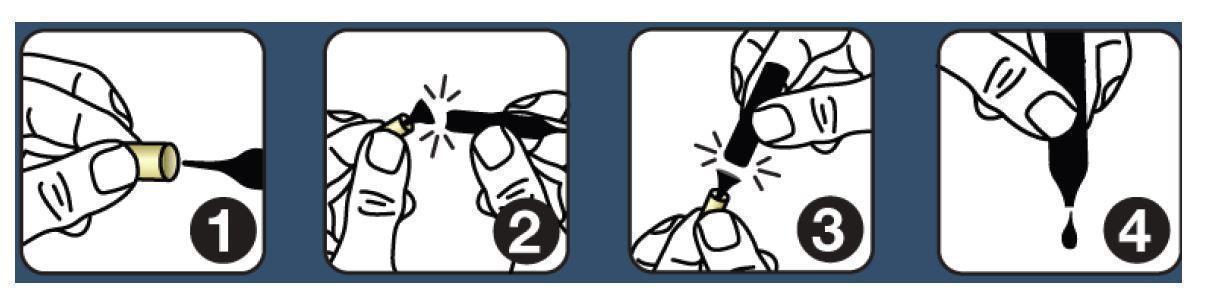
Shake each vial well before use. Follow this protocol:
Days 60-100L 101-180L Day 1 Do not feed the fish
Vial ADo not feed the fish
Vials AB togetherDay 2 Do not feed the fish
Vial B
Do not feed the fish
Vials ADay 3 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 4 Do not feed the fish
Vial AFeed the fish
No TreatmentDay 5 Do not feed the fish Vial B
Feed the fish
No TreatmentDay 6 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 7 Do not feed the fish
Vial ADo not feed the fish
Vials AB at the same time
Day 8 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 9 Water change:
-50% of the volumeFeed the fish
No Treatment
Add the vial ofBioDigest Start
Water Change:
-30% of the volume
Feed the fishNo Treatment
Add the vial of
BioDigest StartEnsure a good flow and good water oxygenation. To protect the biological filter, during the treatment days, remove the bacterial support material from the filter. Run it in another aquarium if possible, or at least keep it moist with water in a container. Reinsert the support material on the days the fish are fed, after briefly rinsing it with clean water.
- - PRESERVATION
-
PRECAUTIONS- CONTRAINDICATIONS & INTERACTIONS
Invertebrates such as snails and shrimps cannot withstand Bacteria & Fungi Fresh, and must be removed from the aquarium before beginning the treatment. Some filiform plants can be damaged by the treatment. The simultaneous use of water treatment products may decrease the effectiveness of Bacteria & Fungi Fresh. Do not use with another treatment.
- - UNWANTED SIDE EFFECTS
- - SPECIAL PRECAUTIONS
- SPL UNCLASSIFIED SECTION
- - Special precautions for the disposal of unused pharmaceuticals
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BACTERIA AND FUNGI AQUARIUM CURE PROGRAM FRESH
acriflavine hydrochloride, methyl orange, ethacridine lactate, methylene blue anhydrous kitProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86048-008 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-008-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 VIAL, SINGLE-USE 3.9 mL Part 2 2 VIAL, SINGLE-USE 2.0 mL Part 1 of 2 A VIAL
acriflavine hydrochloride, methyl orange, ethacridine lactate, methylene blue anhydrous solutionProduct Information Item Code (Source) NDC:86048-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACRIFLAVINE HYDROCHLORIDE (UNII: 1S73VW819C) (ACRIFLAVINE - UNII:1T3A50395T) ACRIFLAVINE 2.00 mg in 1 mL METHYL ORANGE (UNII: 6B4TC34456) (METHYL ORANGE - UNII:6B4TC34456) METHYL ORANGE 0.26 mg in 1 mL ethacridine lactate (UNII: V5IL571C1T) (ETHACRIDINE - UNII:WIX85M1A6R) ethacridine lactate 4.00 mg in 1 mL METHYLENE BLUE ANHYDROUS (UNII: 8NAP7826UB) (METHYLENE BLUE - UNII:T42P99266K) METHYLENE BLUE 2.75 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-009-01 1.3 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Part 2 of 2 B VIAL
acriflavine hydrochloride, methyl orange, ethacridine lactate, methylene blue anhydrous solutionProduct Information Item Code (Source) NDC:86048-014 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACRIFLAVINE HYDROCHLORIDE (UNII: 1S73VW819C) (ACRIFLAVINE - UNII:1T3A50395T) ACRIFLAVINE HYDROCHLORIDE 2.00 mg in 1 mL methyl orange (UNII: 6B4TC34456) (methyl orange - UNII:6B4TC34456) methyl orange 0.26 mg in 1 mL ethacridine lactate (UNII: V5IL571C1T) (ETHACRIDINE - UNII:WIX85M1A6R) ETHACRIDINE 4.00 mg in 1 mL METHYLENE BLUE ANHYDROUS (UNII: 8NAP7826UB) (METHYLENE BLUE - UNII:T42P99266K) METHYLENE BLUE 2.75 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-014-01 1.0 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Labeler - Prodibio SAS (647909431)