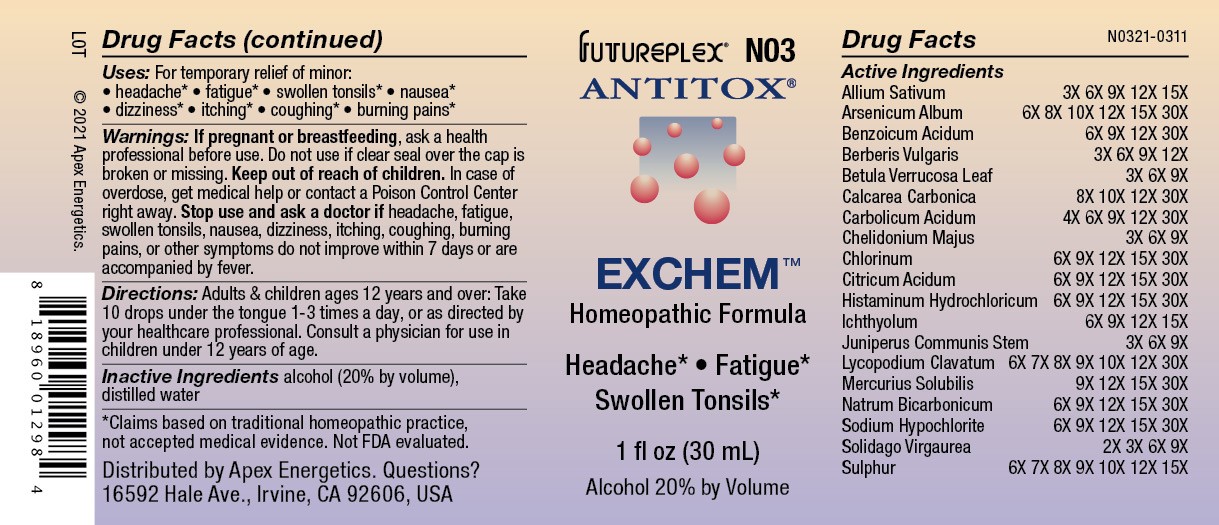
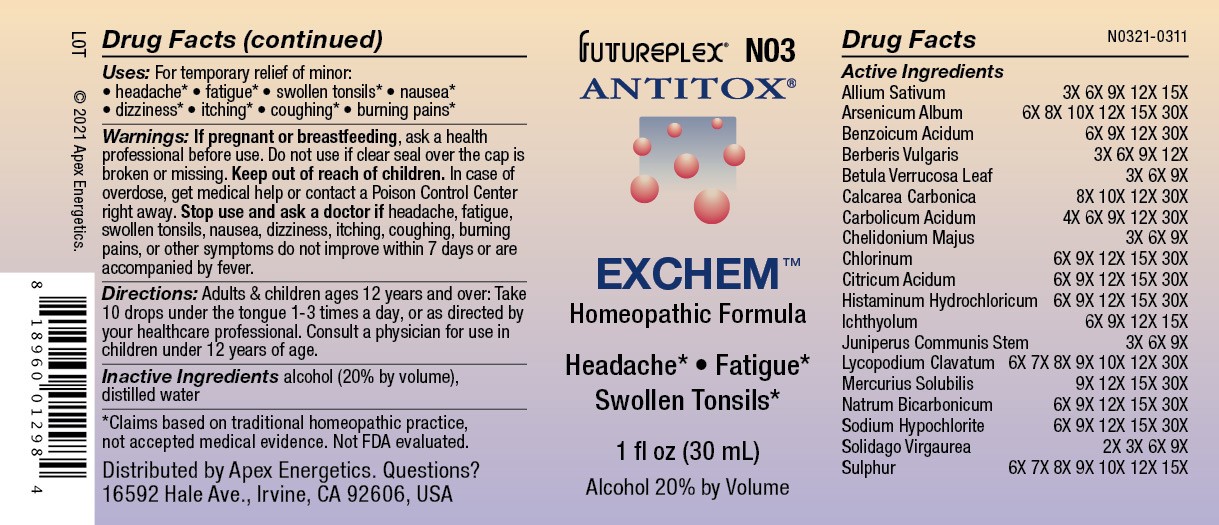
Label: N03 EXCHEM- allium sativum, arsenicum album, benzoicum acidum, berberis vulgaris, betula verrucosa, calcarea carbonica, carbolicum acidum, chelidonium majus, chlorinum, citricum acidum, histaminum, ichthyolum, juniperus communis, lycopodium clavatum, mercurius solubilis, natrum bicarbonicum, sodium hypochlorite, solidago virgaurea, sulphur solution/ drops
- NDC Code(s): 63479-1403-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Allium Sativum
3X 6X 9X 12X 15X
Arsenicum Album
6X 8X 10X 12X 15X 30X
Benzoicum Acidum
6X 9X 12X 30X
Berberis Vulgaris
3X 6X 9X 12X
Betula Verrucosa Leaf
3X 6X 9X
Calcarea Carbonica
8X 10X 12X 30X
Carbolicum Acidum
4X 6X 9X 12X 30X
Chelidonium Majus
3X 6X 9X
Chlorinum
6X 9X 12X 15X 30X
Citricum Acidum
6X 9X 12X 15X 30X
Histaminum Hydrochloricum
6X 9X 12X 15X 30X
Ichthyolum
6X 9X 12X 15X
Juniperus Communis Stem
3X 6X 9X
Lycopodium Clavatum
6X 7X 8X 9X 10X 12X 30X
Mercurius Solubilis
9X 12X 15X 30X
Natrum Bicarbonicum
6X 9X 12X 15X 30X
Sodium Hypochlorite
6X 9X 12X 15X 30X
Solidago Virgaurea
2X 3X 6X 9X
Sulphur
6X 7X 8X 9X 10X 12X 15X
- Uses:
- Warnings:
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
N03 EXCHEM
allium sativum, arsenicum album, benzoicum acidum, berberis vulgaris, betula verrucosa, calcarea carbonica, carbolicum acidum, chelidonium majus, chlorinum, citricum acidum, histaminum, ichthyolum, juniperus communis, lycopodium clavatum, mercurius solubilis, natrum bicarbonicum, sodium hypochlorite, solidago virgaurea, sulphur solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-1403 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength JUNIPERUS COMMUNIS STEM (UNII: HBU87MH6OY) (JUNIPERUS COMMUNIS STEM - UNII:HBU87MH6OY) JUNIPERUS COMMUNIS STEM 9 [hp_X] in 1 mL SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) (HYPOCHLORITE ION - UNII:T5UM7HB19N) SODIUM HYPOCHLORITE 30 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_X] in 1 mL PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 30 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS WHOLE - UNII:7E889U5RNN) CHELIDONIUM MAJUS 9 [hp_X] in 1 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 30 [hp_X] in 1 mL ICHTHAMMOL (UNII: NQ14646378) (ICHTHAMMOL - UNII:NQ14646378) ICHTHAMMOL 15 [hp_X] in 1 mL GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 15 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_X] in 1 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 30 [hp_X] in 1 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 30 [hp_X] in 1 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 9 [hp_X] in 1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 30 [hp_X] in 1 mL BENZOIC ACID (UNII: 8SKN0B0MIM) (BENZOIC ACID - UNII:8SKN0B0MIM) BENZOIC ACID 30 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 12 [hp_X] in 1 mL BETULA PENDULA LEAF (UNII: 5HW39H9KDH) (BETULA PENDULA LEAF - UNII:5HW39H9KDH) BETULA PENDULA LEAF 9 [hp_X] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_X] in 1 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 30 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 15 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-1403-1 1 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 07/15/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/15/1988 Labeler - Apex Energetics Inc. (195816384)