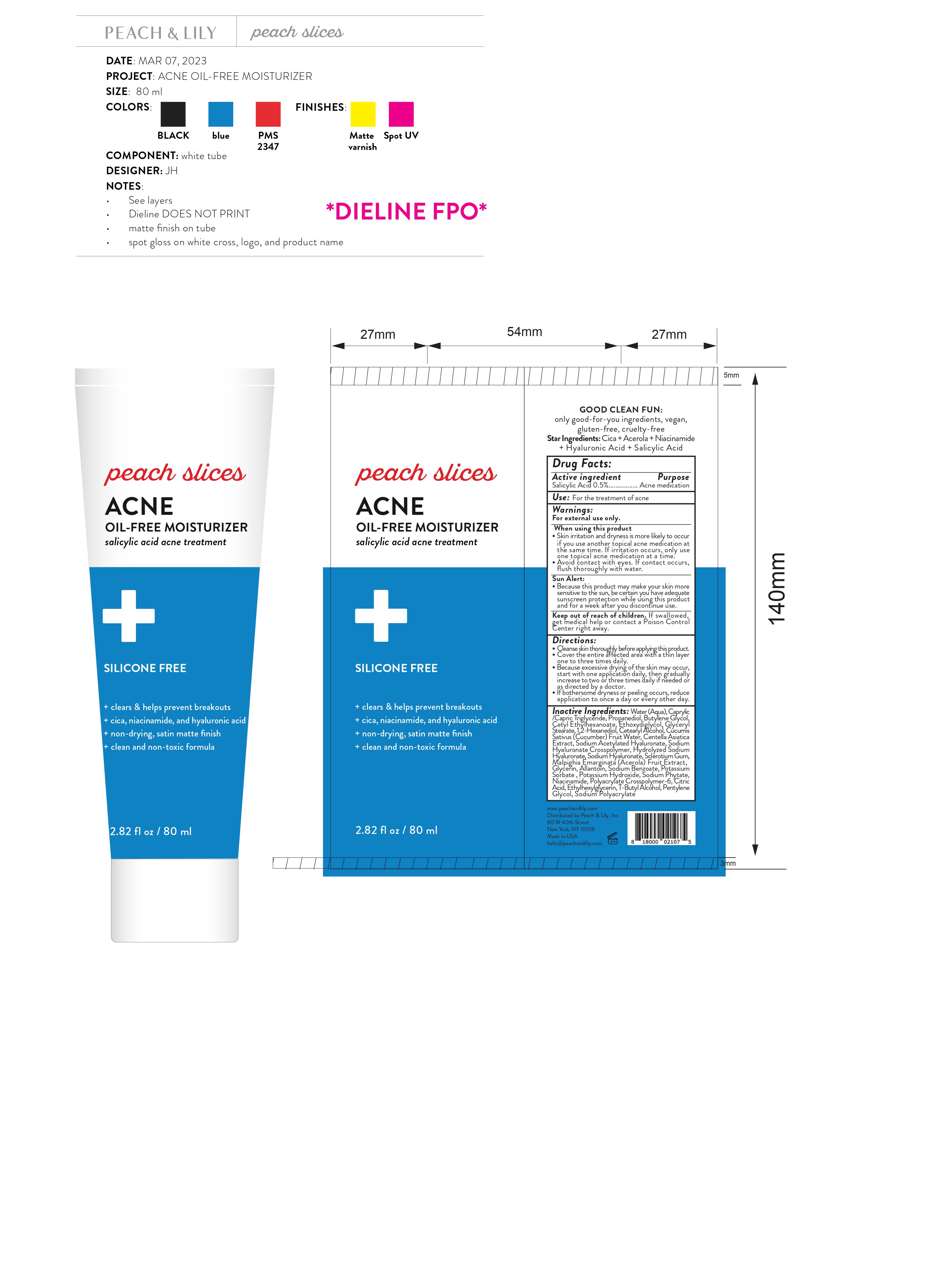
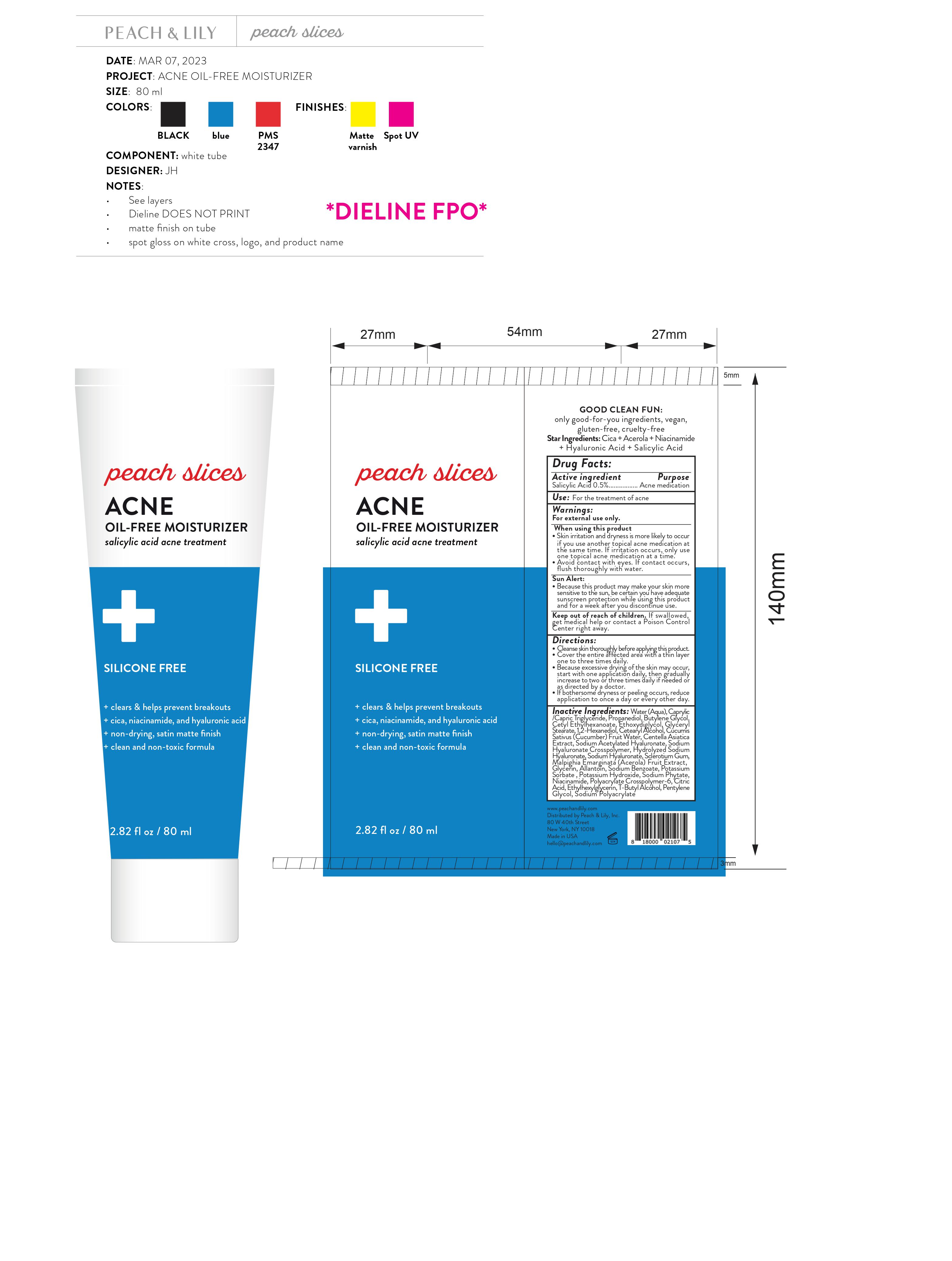
Label: PEACH AND LILY PEACHES SLICES ACNE OIL ACNE FREE MOISTURIZER- salicylic acid gel
- NDC Code(s): 81515-502-01
- Packager: Peach & Lily, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts:
- Active ingredient
- Use:
-
Warnings:
For external use only.
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Avoid contact with eyes. If contact occurs, flush thoroughly with water.
- Because this product may make your skin more sensitive to the sun, be certain you have adequate sunscreen protection while using this product and for a week after you discontinue use.
Sun Alert:
-
Directions:
- Cleanse skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
Inactive Ingredients:
Water (Aqua), Caprylic /Capric Triglyceride, Propanediol, Butylene Glycol, Cetyl Ethylhexanoate, Ethoxydiglycol, Glyceryl Stearate, 1,2-Hexanediol, Cetearyl Alcohol, Cucumis Sativus (Cucumber) Fruit Water, Centella Asiatica Extract, Sodium Acetylated Hyaluronate, Sodium Hyaluronate Crosspolymer, Hydrolyzed Sodium Hyaluronate, Sodium Hyaluronate, Sclerotium Gum, Malpighia Emarginata (Acerola) Fruit Extract, Glycerin, Allantoin, Sodium Benzoate, Potassium Sorbate , Potassium Hydroxide, Sodium Phytate, Niacinamide, Polyacrylate Crosspolymer-6, Citric Acid, Ethylhexylglycerin, T-Butyl Alcohol, Pentylene Glycol, Sodium Polyacrylate
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PEACH AND LILY PEACHES SLICES ACNE OIL ACNE FREE MOISTURIZER
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81515-502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CUCUMBER FRUIT OIL (UNII: R81Y52NPCT) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BETASIZOFIRAN (UNII: 2X51AD1X3T) ACEROLA (UNII: XDD2WEC9L5) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) HEXASODIUM PHYTATE (UNII: ZBX50UG81V) NIACINAMIDE (UNII: 25X51I8RD4) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) PENTYLENE GLYCOL (UNII: 50C1307PZG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81515-502-01 80 mL in 1 TUBE; Type 0: Not a Combination Product 03/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/01/2021 Labeler - Peach & Lily, Inc. (079906552)