Label: EXTRANEAL- icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution
- NDC Code(s): 0941-0707-03, 0941-0707-08
- Packager: Vantive US Healthcare LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 10, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Health Care Provider Letter
 ...
... -
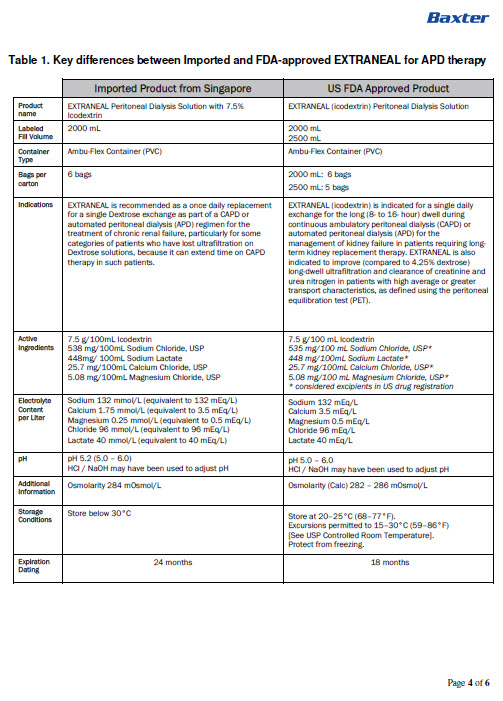
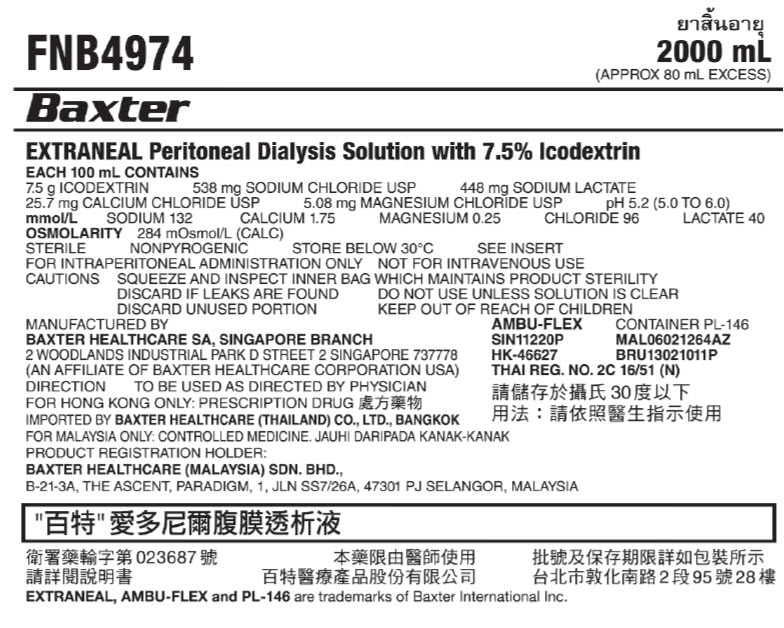
PACKAGE/LABEL PRINCIPAL DISPLAY PANELFNB4974 2000 mL - (APPROX 80 mL EXCESS) BaxterLogo - EXTRANEAL Peritoneal Dialysis Solution with 7.5% lcodextrin - EACH 100 mL CONTAINS - 7.5 g ICODEXTRIN 538 mg SODIUM ...
-
INGREDIENTS AND APPEARANCEProduct Information