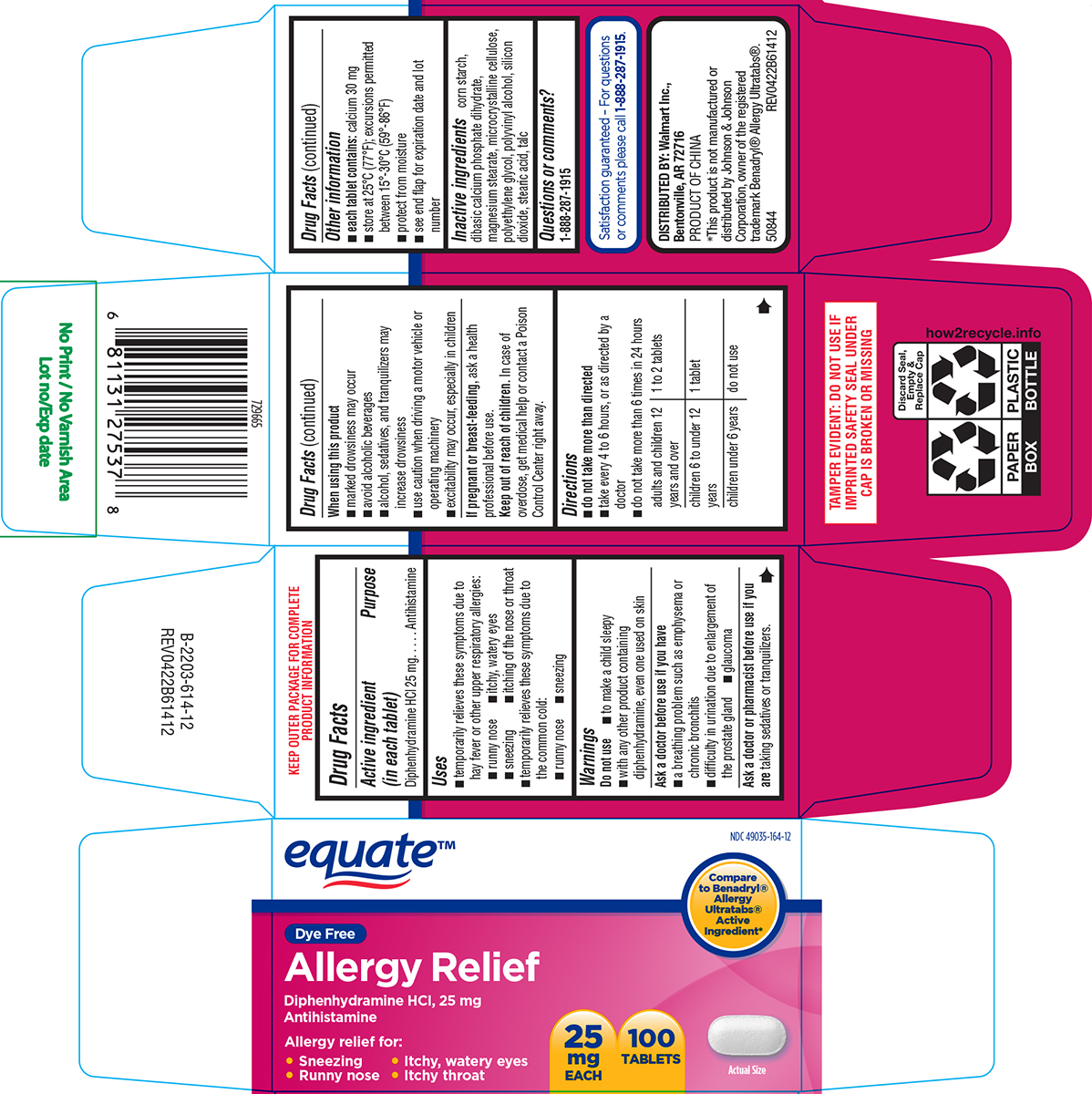
Label: ALLERGY RELIEF- diphenhydramine hci tablet, film coated
- NDC Code(s): 49035-164-12
- Packager: Wal-Mart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
equate™
NDC 49035-164-12
Compare to Benadryl® Allergy Ultratabs® Active Ingredient*
Dye Free
Allergy Relief
Diphenhydramine HCI, 25 mg
AntihistamineAllergy relief for:
• Sneezing • Itchy, watery eyes
• Runny nose • Itchy throat25
mg
EACH100
TABLETSActual Size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSINGSatisfaction guaranteed – For questions
or comments please call 1-888-287-1915.DISTRIBUTED BY: Walmart Inc.,
Bentonville, AR 72716PRODUCT OF CHINA
*This product is not manufactured or
distributed by Johnson & Johnson
Corporation, owner of the registered
trademark Benadryl® Allergy Ultratabs®.
50844 REV0422B61412
Equate 44-614
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hci tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-164 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white Score no score Shape OVAL Size 11mm Flavor Imprint Code 44;614 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-164-12 1 in 1 CARTON 08/15/2018 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/15/2018 Labeler - Wal-Mart Stores Inc (051957769) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(49035-164) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(49035-164) , pack(49035-164) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(49035-164) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(49035-164) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(49035-164)