Label: REPUERA DERMATOZYME 5% GAUZE DRESSING MASK- glycerin, allantoin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81459-301-01 - Packager: ALLUV
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
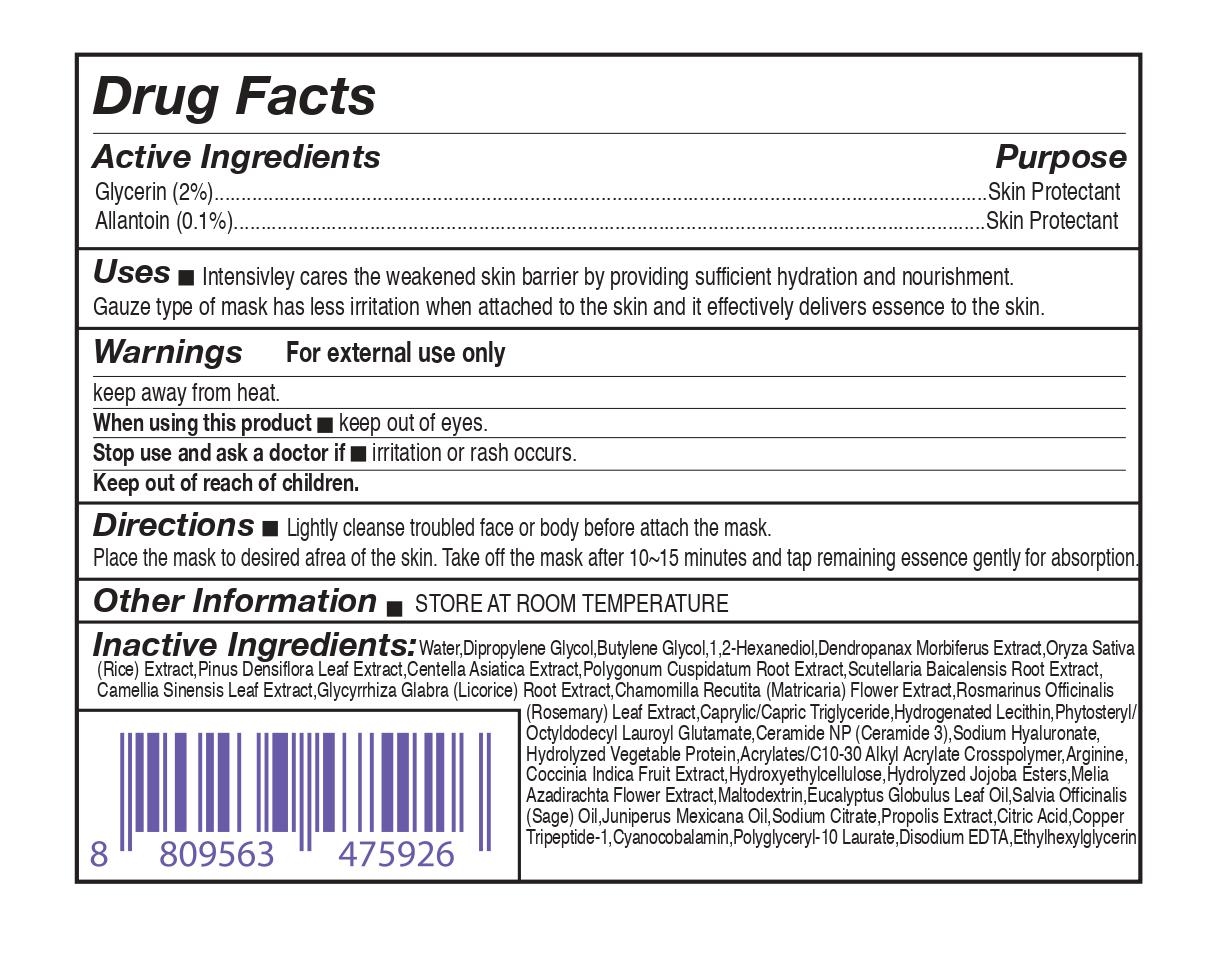
- Active Ingridients
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other Information
-
Inactive Ingredients
Water,Dipropylene Glycol ,Butylene Glycol, 1,2-Hexanediol,Dendropanax Morbiferus Extract,Oryza
Sativa (Rice) Extract,Pinus Densiflora Leaf Extract,Centella Asiatica Extract,Polygonum Cuspidatum Root Extract,Scutellaria Baicalensis Root
Extract,Camellia Sinensis Leaf Extract,Glycyrrhiza Glabra (Licorice) Root Extract,Chamomilla Recutita (Matricaria) Flower Extract,Rosmarinus
Officinalis (Rosemary) Leaf Extract,Caprylic/Capric Triglyceride,Hydrogenated Lecithin,
Phytosteryl/Octyldodecyl Lauroyl Glutamate,Ceramide NP (Ceramide 3),Sodium
Hyaluronate,Hydrolyzed Vegetable Protein,Acrylates/C10-30 Alkyl Acrylate
Crosspolymer,Arginine,Coccinia Indica Fruit Extract,Hydroxyethylcellulose,Hydrolyzed
Jojoba Esters,Melia Azadirachta Flower Extract,Maltodextrin ,Salvia Officinalis (Sage)
Oil,Eucalyptus Globulus Leaf Oil, Juniperus Mexicana Oil,Sodium Citrate,Propolis Extract,Citric Acid,Copper
Tripeptide-1,Cyanocobalamin,Polyglyceryl-10 Laurate,Disodium EDTA,Ethylhexylglycerin - Package Label
-
INGREDIENTS AND APPEARANCE
REPUERA DERMATOZYME 5% GAUZE DRESSING MASK
glycerin, allantoin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81459-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 mg in 100 mL ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GERANIOL (UNII: L837108USY) LINALOOL, (+/-)- (UNII: D81QY6I88E) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ARGININE (UNII: 94ZLA3W45F) MALTODEXTRIN (UNII: 7CVR7L4A2D) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) AZADIRACHTA INDICA FLOWER (UNII: 3TE8A92UPM) SAGE OIL (UNII: U27K0H1H2O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CERAMIDE NP (UNII: 4370DF050B) RICE GERM (UNII: 7N2B70SFEZ) PINUS DENSIFLORA LEAF (UNII: Q1Q9P50WIY) CENTELLA ASIATICA (UNII: 7M867G6T1U) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) ROSEMARY (UNII: IJ67X351P9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCCINIA GRANDIS LEAF (UNII: YJE89UL5PL) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) EUCALYPTUS OIL (UNII: 2R04ONI662) JUNIPERUS DEPPEANA WOOD OIL (UNII: 4739QA5686) SODIUM CITRATE (UNII: 1Q73Q2JULR) PROPOLIS WAX (UNII: 6Y8XYV2NOF) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LIMONENE, (+)- (UNII: GFD7C86Q1W) WATER (UNII: 059QF0KO0R) PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE (UNII: 65954KGO9Q) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PREZATIDE COPPER (UNII: 6BJQ43T1I9) CYANOCOBALAMIN (UNII: P6YC3EG204) DIPROPYLENE GLYCOL (UNII: E107L85C40) POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) CHAMOMILE (UNII: FGL3685T2X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81459-301-01 18 mL in 1 POUCH; Type 0: Not a Combination Product 01/28/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/28/2021 Labeler - ALLUV (695097760) Registrant - ALLUV (695097760) Establishment Name Address ID/FEI Business Operations ALLUV 695097760 manufacture(81459-301)