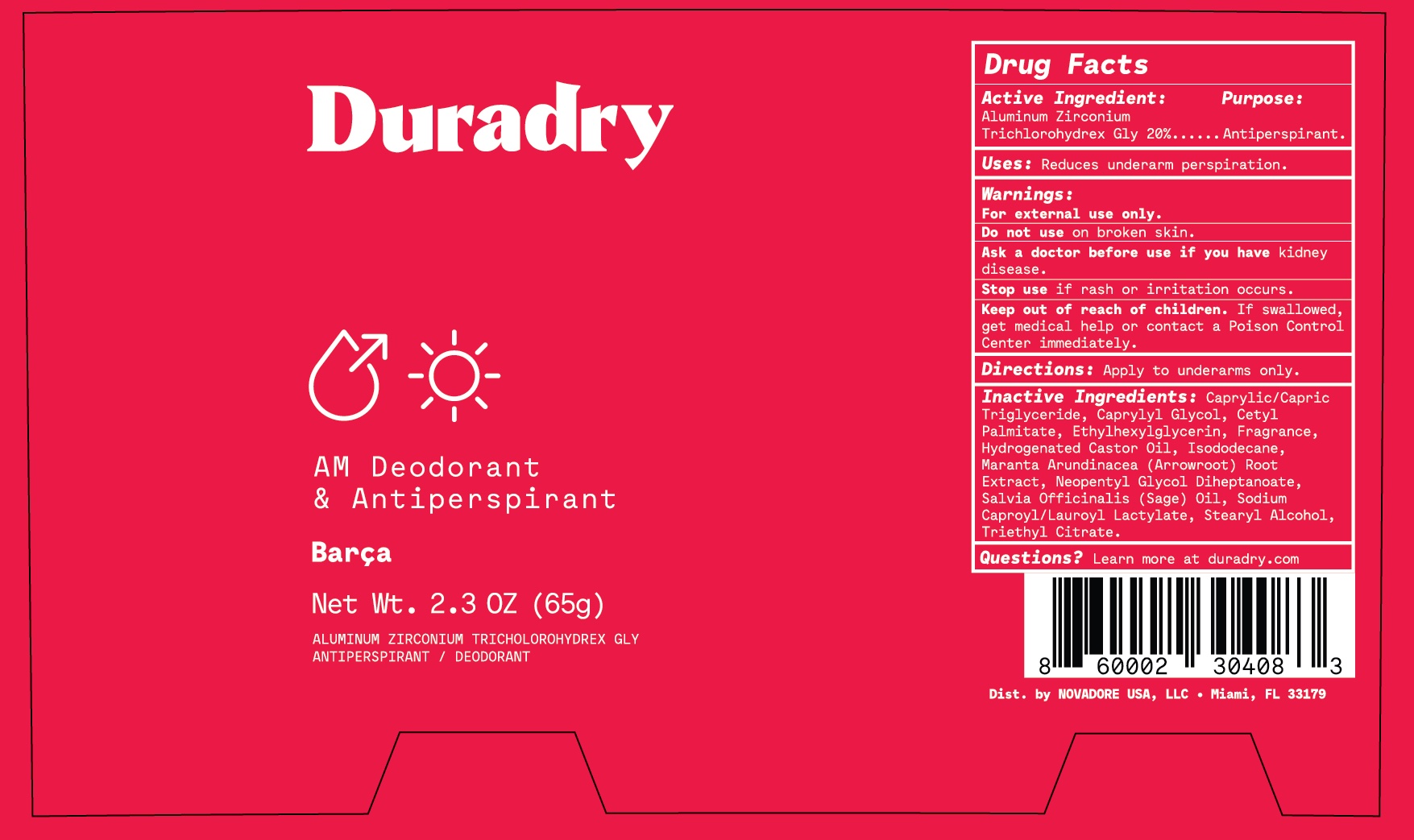
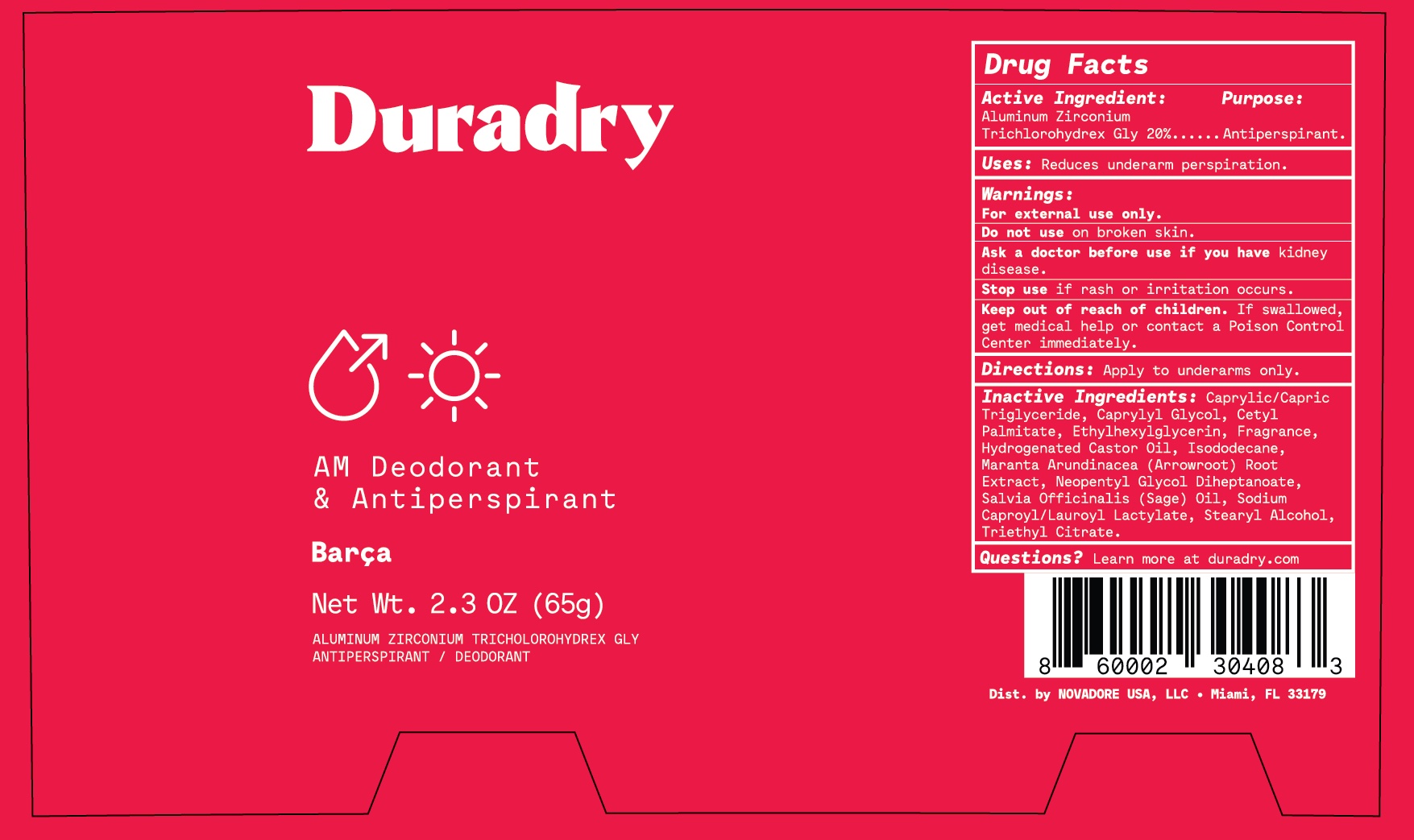
Label: DURADRY AM DEODORANT AND ANTIPERSPIRANT- aluminum zirconium trichlorohydrex glycine stick
- NDC Code(s): 69990-315-01
- Packager: NOVADORE USA Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use
- Keep out of reach of children
- Directions
-
Inactive Ingredients
C13-15 Alkane, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cetyl Palmitate, Ethylhexylglycerin, Fragrance, Hydrogenated Castor Oil, Isododecane, Isohexadecane, Maranta Arundinacea (Arrowroot) Root Extract, Salvia Officinalis (Sage) Oil, Sodium Caproyl/Lauroyl Lactylate, Stearyl Alcohol, Triethyl Citrate.
- Questions?
- DURADRY AM (ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLYCINE)
-
INGREDIENTS AND APPEARANCE
DURADRY AM DEODORANT AND ANTIPERSPIRANT
aluminum zirconium trichlorohydrex glycine stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69990-315 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY (UNII: T27D6T99LH) (ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY - UNII:T27D6T99LH) ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY 20 g in 100 g Inactive Ingredients Ingredient Name Strength ISOHEXADECANE (UNII: 918X1OUF1E) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SALVIA OFFICINALIS ROOT (UNII: 236QY0A1BL) SODIUM CAPROYL LACTYLATE (UNII: 87WR3BHC09) CETYL PALMITATE (UNII: 5ZA2S6B08X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) MARANTA ARUNDINACEA ROOT (UNII: FVN346W31A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) ISODODECANE (UNII: A8289P68Y2) C13-15 ALKANE (UNII: 114P5I43UJ) FRAGRANCE 13576 (UNII: 5EM498GW35) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69990-315-01 1 in 1 CYLINDER 01/27/2021 1 65 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/27/2021 Labeler - NOVADORE USA Inc (079777451) Registrant - NOVADORE USA Inc (079777451)