Label: CYSTARAN- cysteamine hydrochloride solution
- NDC Code(s): 54482-020-01, 54482-020-02, 54482-035-02
- Packager: Leadiant Biosciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYSTARAN® safely and effectively. See full prescribing information for CYSTARAN.
CYSTARAN (cysteamine ophthalmic solution) 0.44%, for topical ophthalmic use
Initial U.S. Approval: 1994INDICATIONS AND USAGE
CYSTARAN is a cystine-depleting agent indicated for the treatment of corneal cystine crystal accumulation in patients with cystinosis. (1)
DOSAGE AND ADMINISTRATION
Instill one drop of CYSTARAN in each eye, every waking hour. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 6.5 mg/mL of cysteamine hydrochloride equivalent to 4.4 mg/mL of cysteamine (0.44%). (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
To minimize the risk of contamination, do not touch the dropper tip to any surface. Keep bottle tightly closed when not in use. (5.1)
ADVERSE REACTIONS
The most common adverse reactions (incidence approximately 10% or greater) are sensitivity to light, redness, eye pain/irritation, headache and visual field defects. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Leadiant Biosciences, Inc. at 1-888-393-4584 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
5.2 Benign Intracranial Hypertension
5.3 Contact Lens Use
5.4 Topical Ophthalmic Use
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
To minimize contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.2 Benign Intracranial Hypertension
There have been reports of benign intracranial hypertension (or pseudotumor cerebri) associated with oral cysteamine treatment that has resolved with the addition of diuretic therapy.
There have also been reports associated with ophthalmic use of cysteamine; however, all of these patients were on concurrent oral cysteamine.
5.3 Contact Lens Use
CYSTARAN contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to application of solution and may be reinserted 15 minutes following its administration [see Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure in controlled clinical trials of six months to 19 years duration in approximately 300 patients.
The most frequently reported ocular adverse reactions occurring in ≥10% of patients were sensitivity to light, redness, and eye pain/irritation, headache and visual field defects.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies of ophthalmic cysteamine in pregnant women to inform any drug associated risks. Oral administration of cysteamine to pregnant rats throughout the period of organogenesis was teratogenic at doses 86 to 345 times the recommended human ophthalmic dose (based on body surface area) [see Data]. CYSTARAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Data
Animal Data
Teratology studies have been performed in rats at oral doses in the range of 37.5 mg/kg/day to 150 mg/kg/day (86 to 345 times the recommended human ophthalmic dose based on a body surface area) and have revealed cysteamine bitartrate to be teratogenic. Observed teratogenic findings were intrauterine death, cleft palate, kyphosis, heart ventricular septal defects, microcephaly, exencephaly, and growth deficits.
8.2 Lactation
Risk Summary
There is no information regarding the presence of cysteamine in human milk, the effects on the breastfed infants, or the effects on milk production. Cysteamine administered orally is present in milk of lactating rats. It is not known whether measurable levels of cysteamine would be present in maternal milk following topical ocular administration of CYSTARAN.
8.4 Pediatric Use
The safety and effectiveness of CYSTARAN (cysteamine ophthalmic solution) 0.44% have been established in pediatric patients.
8.5 Geriatric Use
When the clinical studies with CYSTARAN were conducted, the reduced life expectancy from cystinosis did not make it possible to include patients in the geriatric age range.
8.6 Renal Impairment
The effect of renal impairment on the pharmacokinetics of cysteamine following ophthalmic administration of cysteamine ophthalmic solution has not been evaluated because ophthalmic exposure compared to systemic exposure is negligible. The majority of the patients in the ophthalmic clinical studies are assumed to have had some degree of renal impairment due to their underlying systemic disease. The total daily ophthalmic dose is less than 2% of the recommended oral daily dose of cysteamine; thus, the systemic exposure following ophthalmic administration is expected to be negligible compared to oral administration.
-
11 DESCRIPTION
CYSTARAN is a sterile ophthalmic solution containing 6.5 mg/mL of cysteamine hydrochloride, equivalent to 4.4 mg/mL of cysteamine (0.44%) as the active ingredient. Cysteamine is a cystine-depleting agent which lowers the cystine content of cells in patients with cystinosis.
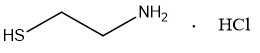
Molecular Formula: C2H7NS HCl
Molecular Weight: 113.61Each milliliter of CYSTARAN contains: Active: cysteamine 4.4 mg (equivalent to cysteamine hydrochloride 6.5 mg); Preservative: benzalkonium chloride 0.1 mg; Inactive Ingredients: sodium chloride, hydrochloric acid and/or sodium hydroxide (to adjust pH to 4.1-4.5), and purified water.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Cysteamine has not been tested for its carcinogenic potential in long-term animal studies.
Mutagenesis
Cysteamine was not mutagenic in the Ames test. It produced a negative response in an in vitro sister chromatid exchange assay in human lymphocytes but a positive response in a similar assay in hamster ovarian cells.
Impairment of Fertility
Repeat breeding reproduction studies were conducted in male and female rats. Cysteamine was found to have no effect on fertility and reproductive performance at an oral dose of 75 mg/kg/day (173 times the recommended human ophthalmic dose based on body surface area). At an oral dose of 375 mg/kg/day (864 times the recommended human ophthalmic dose based on body surface area), it reduced the fertility of the adult rats and the survival of their offspring.
-
14 CLINICAL STUDIES
Clinical efficacy was evaluated in controlled clinical trials in approximately 300 patients. The primary efficacy end point was the response rate of eyes that had a reduction of at least 1 unit in the photo-rated Corneal Cystine Crystal Score (CCCS) at some time point during the study when baseline CCCS ≥1, or a lack of an increase of more than 1 unit in CCCS throughout the study when baseline CCCS <1.
Study 1 combined the data from three smaller studies. For eyes with a lower baseline of CCCS <1, the response rate was 13% (4/30) [95% CI: (4, 32)]. For eyes with a higher baseline of CCCS ≥1, the response rate was 32% (94/291) [95% CI: (27, 38)].
Study 2 evaluated ocular cystinosis patients who had a baseline of CCCS ≥1. The response rate was 67% (10/15) [95% CI: (38, 88)].
Study 3 also evaluated ocular cystinosis patients; for eyes with a baseline of CCCS ≥1, the response rate was 33% (3/9) [95% CI: (8, 70)].
Corneal crystals accumulate if CYSTARAN is discontinued.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fifteen (15) mL of CYSTARAN (cysteamine ophthalmic solution) 0.44% is supplied in an opaque, white, low-density polyethylene (LDPE) 88 mm tall bottle with a 1.6 mm blue, silicone rubber flow-controlled dropper tip and closed with a white, high-density polyethylene (HDPE) screw cap. The bottle is foil-wrapped and stored in a carton.
CYSTARAN bottles must be stored in the following conditions:
NDC 54482-020-02
- Before Opening: Store unopened bottle in freezer at -25°C to -15°C (-13°F to 5°F) in the original packaging including unopened foil. Thaw for approximately 24 hours before use.
- Open the carton and the foil only when starting a new bottle.
- After Opening: Record discard date on the bottle, which is 1 week from the day the foil and bottle were opened. Store thawed bottle at 2°C to 25°C (36°F to 77°F) for up to 1 week. The thawed bottle does not require refrigeration between use. Do not refreeze the thawed medication. Discard 1 week after the foil and bottle were opened even if there is medication left in the bottle.
NDC 54482-035-02
- Before Opening: Store unopened bottle in refrigerator at 2°C to 8°C (36°F to 46°F) in the original packaging including unopened foil.
- Open the carton and the foil only when starting a new bottle.
- After Opening: Record discard date on the bottle, which is 1 week from the day the foil and bottle were opened. During the week of use, store bottle at room temperature, 20°C to 25°C (68°F to 77°F). Discard 1 week after the foil and bottle were opened even if there is medication left in the bottle.
-
17 PATIENT COUNSELING INFORMATION
Storage of Cystaran Bottles NDC 54482-020-02
- Patients should be advised to store unopened bottles in the freezer in the intact foil pouch and original carton.
- Each week, one new bottle should be removed from the freezer.
- Patients should be advised to allow the bottle to thaw completely (approximately 24 hours) prior to use.
- After the bottle is completely thawed, the patient should be advised to open the packaging (carton and foil) and record the discard date on the bottle label. The discard date is seven (7) days from the day the foil and bottle were opened.
- During the week of use, patients should be advised to store thawed bottle at 2°C to 25°C (36°F to 77°F) for up to 1 week. The thawed bottle does not require refrigeration between use and should not be refrozen.
- At the end of 1 week (7 days), patients should discard the bottle. There may be medication left in the bottle; however, the bottle must be discarded by the patient because the medication is only stable for 1 week after the foil and bottle were opened.
Storage of Cystaran Bottles NDC 54482-035-02
- Patients should be advised to store unopened bottles in the refrigerator in the intact foil pouch and original carton.
- When starting a new bottle, patients should be advised to open the packaging (carton and foil) and record the discard date on the bottle label. The discard date is seven (7) days from the day the foil and bottle were opened.
- During the week of use, patients should be advised to store the bottle at room temperature 20°C to 25°C (68°F to 77°F) for up to 1 week.
- At the end of 1 week (7 days), patients should discard the bottle. There may be medication left in the bottle; however, the bottle must be discarded by the patient because the medication is only stable for 1 week after the foil and bottle were opened.
Risk of Contamination
Patients should be advised not to touch the eyelid or surrounding areas with the dropper tip of the bottle. The cap should remain on the bottle when not in use.
- SPL UNCLASSIFIED SECTION
-
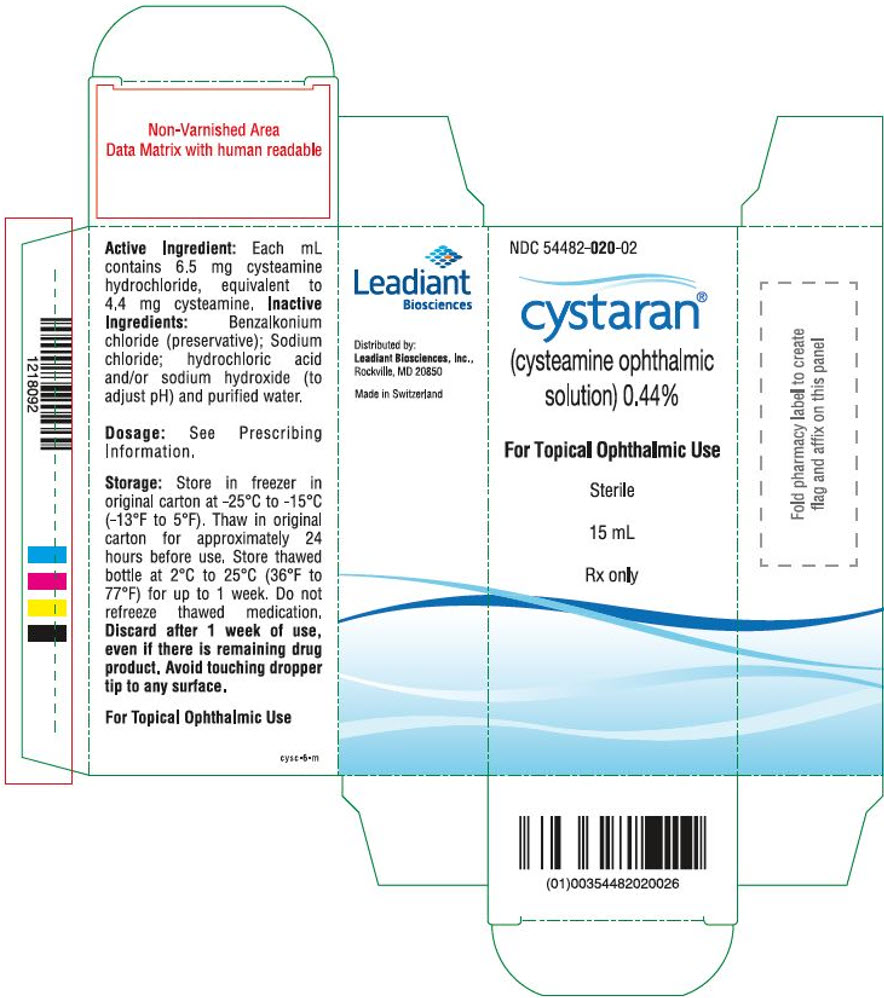
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
NDC 54482-020-02
cystaran®
(cysteamine ophthalmic
solution) 0.44%For Topical Ophthalmic Use
Sterile
15 mL
Rx only
Active Ingredient: Each mL
contains 6.5 mg cysteamine
hydrochloride, equivalent to
4.4 mg cysteamine. Inactive
Ingredients: Benzalkonium
chloride (preservative); Sodium
chloride; hydrochloric acid
and/or sodium hydroxide (to
adjust pH) and purified water.Dosage: See Prescribing
Information.Storage: Store in freezer in
original carton at -25°C to -15°C
(-13°F to 5°F). Thaw in original
carton for approximately 24
hours before use. Store thawed
bottle at 2°C to 25°C (36°F to
77°F) for up to 1 week. Do not
refreeze thawed medication.
Discard after 1 week of use,
even if there is remaining drug
product. Avoid touching dropper
tip to any surface.For Topical Ophthalmic Use
cysc-6-m
Distributed by:
Leadiant Biosciences Inc.,
Rockville, MD 20850Made in Switzerland

-
PRINCIPAL DISPLAY PANEL - Carton
NDC 54482-035-02
cystaran®
(cysteamine ophthalmic
solution) 0.44%For Topical Ophthalmic Use
Sterile
NEW STORAGE
Rx only
15 mL
Active Ingredient: Each mL
contains 6.5 mg cysteamine
hydrochloride, equivalent to
4.4 mg cysteamine.Inactive Ingredients: Benzalko-
nium chloride (preservative);
Sodium chloride; hydrochloric
acid and/or sodium hydroxide (to
adjust pH) and purified water.Dosage: See Prescribing
Information.Storage:
Before Opening: Store in
refrigerator in original packaging,
including unopened foil, at 2°C to
8°C (36°F to 46°F).After Opening: After opening the
foil, store bottle at room
temperature 20°C to 25°C (68°F
to 77°F) during week of use.Discard the bottle 1 week after
opening the foil and bottle, even
if there is remaining medication
in the bottle. Avoid touching
dropper tip to any surface.cysc-7-m
Distributed by:
Leadiant Biosciences, Inc.,
Rockville, MD 20850Made in Switzerland

-
INGREDIENTS AND APPEARANCE
CYSTARAN
cysteamine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54482-020 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYSTEAMINE HYDROCHLORIDE (UNII: IF1B771SVB) (CYSTEAMINE - UNII:5UX2SD1KE2) CYSTEAMINE HYDROCHLORIDE 6.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54482-020-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/2012 06/30/2021 2 NDC:54482-020-02 15 mL in 1 BOTTLE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 02/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200740 12/30/2012 CYSTARAN
cysteamine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54482-035 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYSTEAMINE HYDROCHLORIDE (UNII: IF1B771SVB) (CYSTEAMINE - UNII:5UX2SD1KE2) CYSTEAMINE HYDROCHLORIDE 6.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54482-035-02 15 mL in 1 BOTTLE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 04/25/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200740 12/30/2012 Labeler - Leadiant Biosciences, Inc. (068301431)