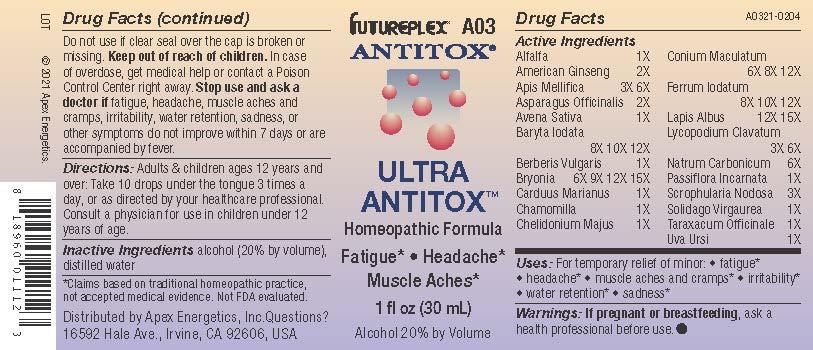
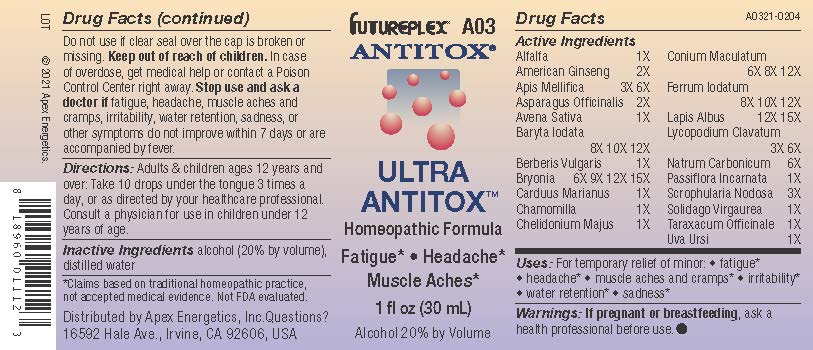
Label: A03 ULTRA ANTITOX- alfalfa, american ginseng, apis mellifica, asparagus officinalis, avena sativa, baryta iodata, berberis vulgaris, bryonia, carduus marianus, chamomilla, chelidonium majus, conium maculatum, ferrum iodatum, lapis albus, lycopodium clavatum, natrum carbonicum, passiflora incarnata, scrophularia nodosa, solidago virgaurea, taraxacum officinale, uva ursi solution/ drops
- NDC Code(s): 63479-0103-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Alfalfa
1X
American Ginseng
2X
Apis Mellifica
3X 6X
Asparagus Officinalis
2X
Avena Sativa
1X
Baryta Iodata
8X 10X 12X
Berberis Vulgaris
1X
Bryonia
6X 9X 12X 15X
Carduus Marianus
1X
Chamomilla
1X
Chelidonium Majus
1X
Conium Maculatum
6X 8X 12X
Ferrum Iodatum
8X 10X 12X
Lapis Albus
12X 15X
Lycopodium Clavatum
3X 6X
Natrum Carbonicum
6X
Passiflora Incarnata
1X
Scrophularia Nodosa
3X
Solidago Virgaurea
1X
Taraxacum Officinale
1X
Uva Ursi
1X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
A03 ULTRA ANTITOX
alfalfa, american ginseng, apis mellifica, asparagus officinalis, avena sativa, baryta iodata, berberis vulgaris, bryonia, carduus marianus, chamomilla, chelidonium majus, conium maculatum, ferrum iodatum, lapis albus, lycopodium clavatum, natrum carbonicum, passiflora incarnata, scrophularia nodosa, solidago virgaurea, taraxacum officinale, uva ursi solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0103 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 15 [hp_X] in 1 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 1 mL ASPARAGUS (UNII: Z1EJP3037Z) (ASPARAGUS - UNII:Z1EJP3037Z) ASPARAGUS 2 [hp_X] in 1 mL AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 1 [hp_X] in 1 mL ALFALFA (UNII: DJO934BRBD) (ALFALFA - UNII:DJO934BRBD) ALFALFA 1 [hp_X] in 1 mL AMERICAN GINSENG (UNII: 8W75VCV53Q) (AMERICAN GINSENG - UNII:8W75VCV53Q) AMERICAN GINSENG 2 [hp_X] in 1 mL FERROUS IODIDE (UNII: F5452U54PN) (FERROUS IODIDE - UNII:F5452U54PN) FERROUS IODIDE 12 [hp_X] in 1 mL SODIUM CARBONATE (UNII: 45P3261C7T) (CARBONATE ION - UNII:7UJQ5OPE7D) SODIUM CARBONATE 6 [hp_X] in 1 mL BARIUM IODIDE (UNII: WKC4T7680A) (BARIUM IODIDE - UNII:WKC4T7680A) BARIUM IODIDE 12 [hp_X] in 1 mL PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 1 [hp_X] in 1 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_X] in 1 mL SCROPHULARIA NODOSA (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA 3 [hp_X] in 1 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 1 [hp_X] in 1 mL CALCIUM HEXAFLUOROSILICATE (UNII: 2NVP93XVQ3) (CALCIUM HEXAFLUOROSILICATE - UNII:2NVP93XVQ3) CALCIUM HEXAFLUOROSILICATE 15 [hp_X] in 1 mL ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) (ARCTOSTAPHYLOS UVA-URSI LEAF - UNII:3M5V3D1X36) ARCTOSTAPHYLOS UVA-URSI LEAF 1 [hp_X] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 1 [hp_X] in 1 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 1 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 1 [hp_X] in 1 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 1 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 1 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0103-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/15/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/15/1994 Labeler - Apex Energetics Inc. (195816384)