Label: SPOTS AND VELVETS AQUARIUM CURE PROGRAM FRESH- acriflavine hydrochloride, gentian violet, malachite green oxalate, methylene blue kit
- NDC Code(s): 86048-004-01, 86048-005-01, 86048-012-01
- Packager: Prodibio SAS
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 3, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INSTRUCTIONS
- PRESENTATION
- SPL UNCLASSIFIED SECTION
-
- ACTIVE INGREDIENTS
The vials A and B contain per mL:
• 0.3 mg of 3,6-Diamino-10-methylacridinium chloride mixt. with 3,6-diaminoacridine (acriflavine chloride)
• 0.5 mg of Hexamethylpararosaniline chloride (crystal violet)
• 1.0 mg of N,N,N',N'-Tetramethyl-4,4'-diaminotriphenylcarbenium oxalate (malachite green oxalate)
• 2.5 mg of 3,7-bis(Dimethylamino)phenazathionium chloride (methylene blue)
The BioDigest Start vial contains nitrifying and denitrifying living bacteria that have not been genetically modified. - - EXCIPIENTS
- - PHARMACEUTICAL FORM & CONTENTS
- - MANUFACTURER
-
USAGE
- THERAPEUTIC INDICATIONS
Product intended only for the treatment of ornamental fish with the so-called "white spots" and "velvet" diseases, mainly caused by Ichthyophthirius, Oodinium and secondary bacterial infections caused indirectly by these protists. Visible symptoms are white spots, granular lesions or fluffy layers of pale gray. They appear on the gills, fins and may extend to the whole body. Infected fish fins retract and try to remove the parasites by rubbing against the scenery. Those whose gills are very affected have an abnormally high respiratory activity, become apathetic and lose weight quickly. -
- DOSAGE AND DIRECTIONS FOR USE
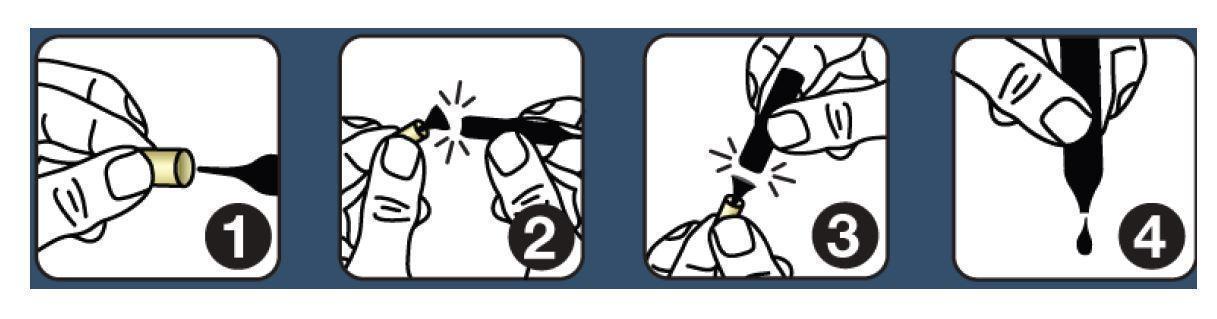
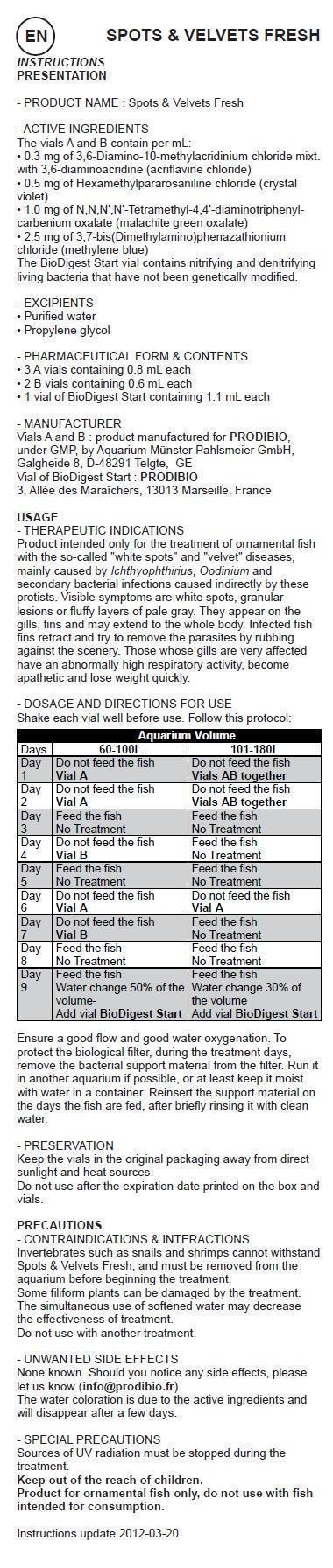
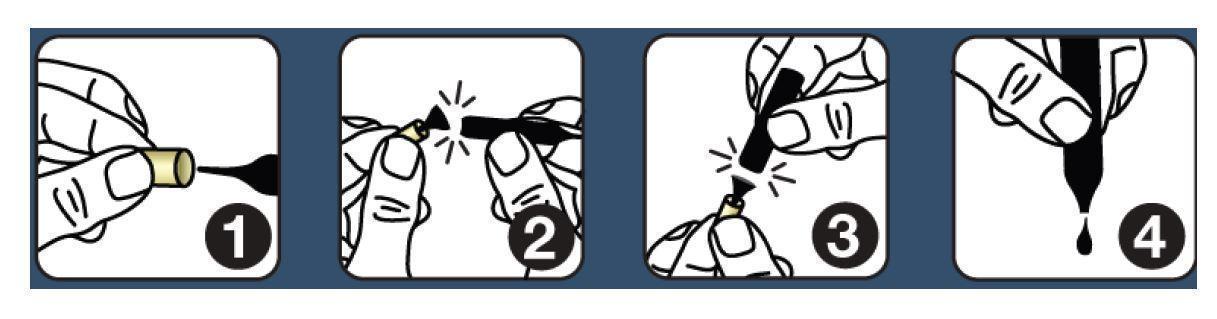
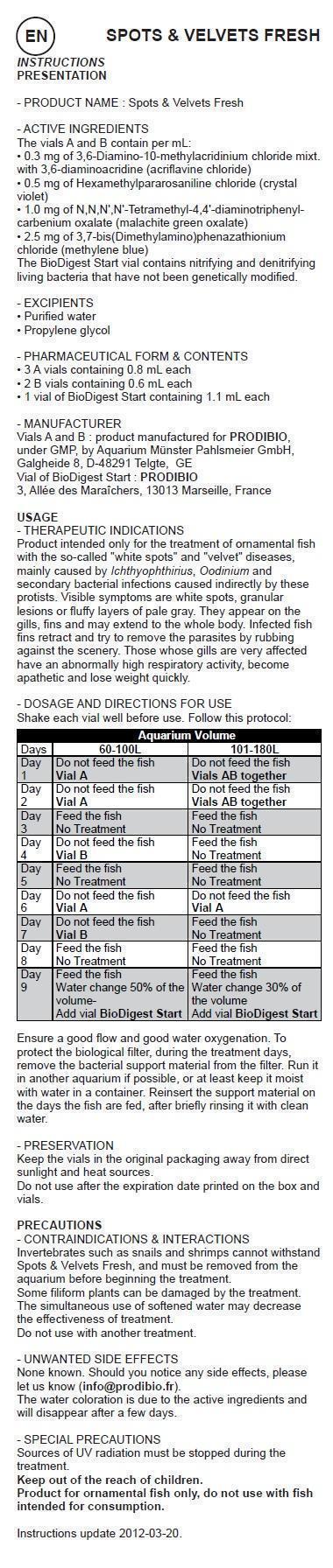
Shake each vial well before use. Follow this protocol:
Aquarium Volume Days 60-100L 101-180L Day 1 Do not feed the fish
Vial ADo not feed the fish
Vials AB togetherDay 2 Do not feed the fish
Vial A
Do not feed the fish
Vials AB togetherDay 3 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 4 Do not feed the fish
Vial BFeed the fish
No TreatmentDay 5 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 6 Do not feed the fish Vial A
Do not feed the fish Vial A
Day 7 Do not feed the fish
Vial BFeed the fish
No TreatmentDay 8 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 9 Feed the fish
Water change 50% of the
volume-
Add the vial BioDigest StartFeed the fish
Water change 30% of
the volume
Add vial BioDigest StartEnsure a good flow and good water oxygenation. To protect the biological filter, during the treatment days, remove the bacterial support material from the filter. Run it in another aquarium if possible, or at least keep it moist with water in a container. Reinsert the support material on the days the fish are fed, after briefly rinsing it with clean water.
- - PRESERVATION
-
PRECAUTIONS- CONTRAINDICATIONS & INTERACTIONS
Invertebrates such as snails and shrimps cannot withstand Spots & Velvets Fresh, and must be removed from the aquarium before beginning the treatment.
Some filiform plants can be damaged by the treatment.
The simultaneous use of softened water may decrease the effectiveness of treatment.
Do not use with another treatment. - - UNWANTED SIDE EFFECTS
- - SPECIAL PRECAUTIONS
- SPL UNCLASSIFIED SECTION
- - Special precautions for the disposal of unused pharmaceuticals
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SPOTS AND VELVETS AQUARIUM CURE PROGRAM FRESH
acriflavine hydrochloride, gentian violet, malachite green oxalate, methylene blue kitProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86048-004 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-004-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 VIAL, SINGLE-USE 2.4 mL Part 2 2 VIAL, SINGLE-USE 1.2 mL Part 1 of 2 A VIAL
acriflavine hydrochloride, gentian violet, malachite green oxalate, methylene blue solutionProduct Information Item Code (Source) NDC:86048-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GENTIAN VIOLET (UNII: J4Z741D6O5) (GENTIAN VIOLET CATION - UNII:3GVJ31T6YY) GENTIAN VIOLET 0.5 mg in 1 mL MALACHITE GREEN OXALATE (UNII: GY1H9O33VU) (MALACHITE GREEN - UNII:12058M7ORO) MALACHITE GREEN 1.0 mg in 1 mL ACRIFLAVINE HYDROCHLORIDE (UNII: 1S73VW819C) (ACRIFLAVINE - UNII:1T3A50395T) ACRIFLAVINE 0.3 mg in 1 mL METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-005-01 0.8 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Part 2 of 2 B VIAL
acriflavine hydrochloride, gentian violet, malachite green oxalate, methylene blue solutionProduct Information Item Code (Source) NDC:86048-012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACRIFLAVINE HYDROCHLORIDE (UNII: 1S73VW819C) (ACRIFLAVINE - UNII:1T3A50395T) ACRIFLAVINE 0.3 mg in 1 mL GENTIAN VIOLET (UNII: J4Z741D6O5) (GENTIAN VIOLET CATION - UNII:3GVJ31T6YY) GENTIAN VIOLET 0.5 mg in 1 mL MALACHITE GREEN OXALATE (UNII: GY1H9O33VU) (MALACHITE GREEN - UNII:12058M7ORO) MALACHITE GREEN 1.0 mg in 1 mL METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-012-01 0.6 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Labeler - Prodibio SAS (647909431)