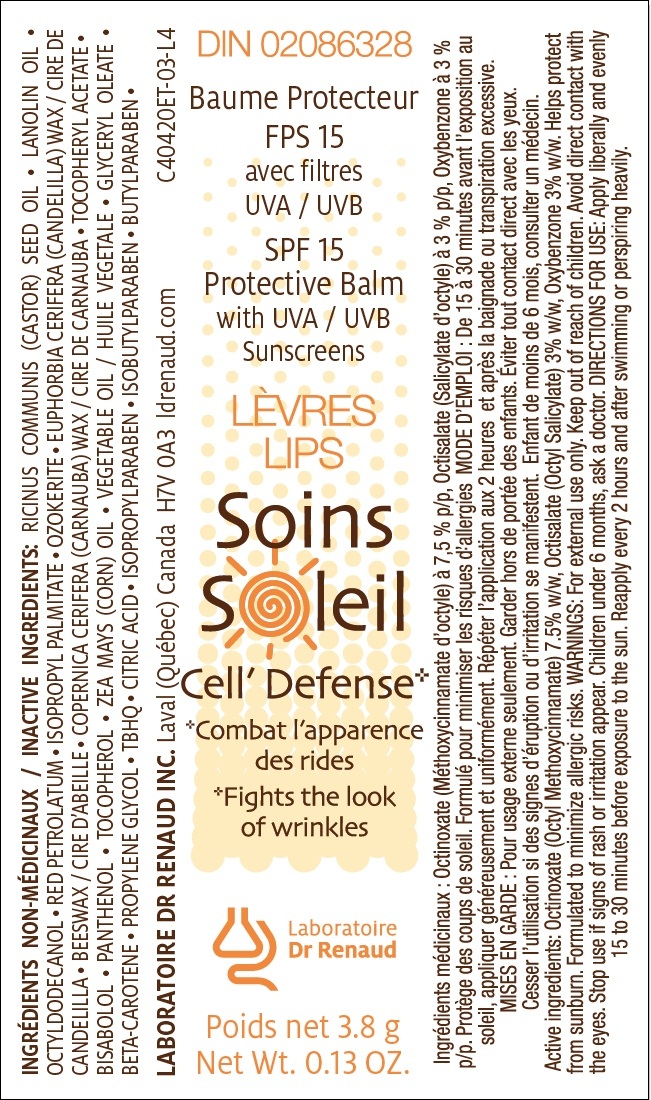
Label: SPF 15 PROTECTIVE BALM WITH UVA/UVB SUNSCREENS- octinoxate octisalate oxybenzone stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 62499-420-02, 62499-420-11 - Packager: Laboratoire Dr Renaud inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 23, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Octinoxate (Octyl Methoxycinnamate) à 7.5 % w/w, Octisalate (Octyl Salicylate) à 3% w/w, Oxybenzone à 3% w/w.
Inactive ingredients
RICINUS COMMUNIS (CASTOR) SEED OIL • LANOLIN OIL • OCTYLDODECANOL • RED PETROLATUM • ISOPROPYL PALMITATE • OZOKERITE • EUPHORBIA CERIFERA (CANDELILLA) WAX / CIRE DE CANDELILLA • BEESWAX / CIRE D’ABEILLE • COPERNICA CERIFERA (CARNAUBA) WAX / CIRE DE CARNAUBA • TOCOPHERYL ACETATE • BISABOLOL • PANTHENOL • TOCOPHEROL • ZEA MAYS (CORN) OIL • VEGETABLE OIL / HUILE VEGETALE • GLYCERYL OLEATE • BETA-CAROTENE • PROPYLENE GLYCOL • TBHQ • CITRIC ACID • ISOPROPYLPARABEN • ISOBUTYLPARABEN • BUTYLPARABEN •
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPF 15 PROTECTIVE BALM WITH UVA/UVB SUNSCREENS
octinoxate octisalate oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62499-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength RICINUS COMMUNIS SEED (UNII: 7EK4SFN1TX) LANOLIN (UNII: 7EV65EAW6H) OCTYLDODECANOL (UNII: 461N1O614Y) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CARNAUBA WAX (UNII: R12CBM0EIZ) ACETATE ION (UNII: 569DQM74SC) LEVOMENOL (UNII: 24WE03BX2T) PANTHENOL (UNII: WV9CM0O67Z) ISOPROPYLPARABEN (UNII: A6EOX47QK0) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) BUTYLPARABEN (UNII: 3QPI1U3FV8) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) CORN OIL (UNII: 8470G57WFM) SUNFLOWER OIL (UNII: 3W1JG795YI) T-BUTYLHYDROQUINONE (UNII: C12674942B) GLYCERYL MONOOLEATE (UNII: 4PC054V79P) BETA CAROTENE (UNII: 01YAE03M7J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color red (dark red) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62499-420-11 1 in 1 BOX 1 NDC:62499-420-02 3.8 g in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/21/2011 Labeler - Laboratoire Dr Renaud inc. (202501565) Registrant - Laboratoire Dr Renaud inc (202501565) Establishment Name Address ID/FEI Business Operations Laboratoire Dr Renaud inc 202501565 manufacture