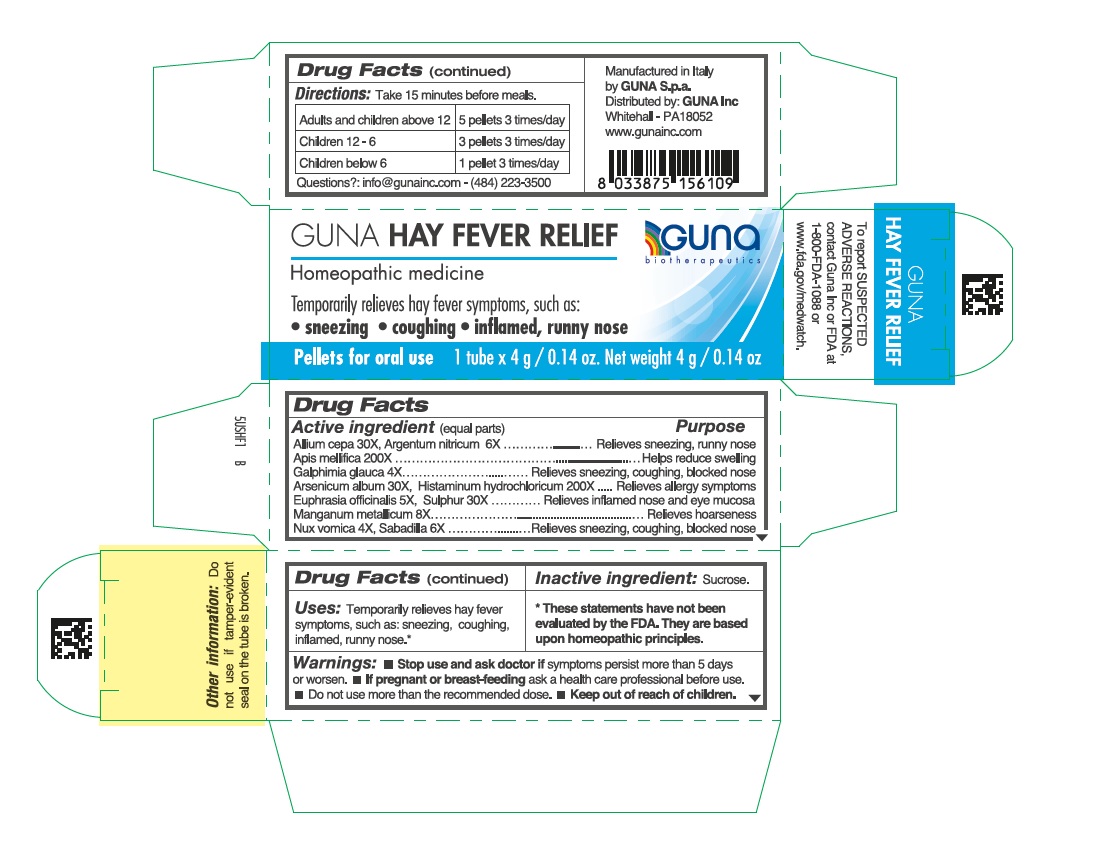
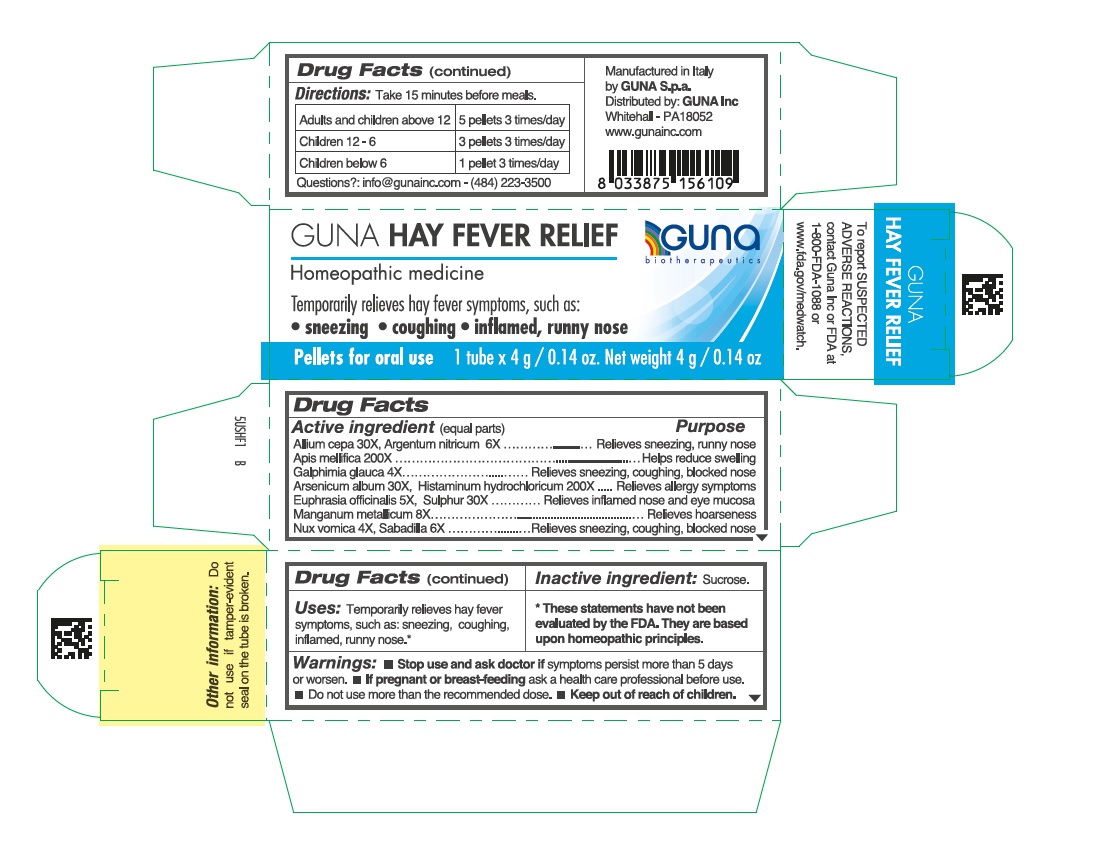
Label: GUNA HAY FEVER RELIEF- strychnos nux vomica seed - histamine dihydrochloride - manganese - schoenocaulon officinale seed - apis mellifera - arsenic trioxide - silver nitrate - onion - euphrasia stricta - galphimia glauca flowering top - sulfur - pellet
- NDC Code(s): 17089-476-20, 17089-476-21
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- QUESTIONS
- WARNINGS
- PREGNANCY
- USES
- WARNINGS
- WARNINGS
-
ACTIVE INGREDIENTS/PURPOSE
Allium cepa 30X Relieves sneezing, runny nose
Apis mellifica 200X Helps reduce swelling
Galphimia glauca 4X Relieves sneezing, coughing, blocked nose
Argentum nitricum 6X Relieves sneezing, runny nose
Arsenicum album 30X Relieves allergy symptoms
Euphrasia officinalis 5X Relieves inflamed nose and eye mucosa
Histaminum hydrochloricum 200X Relieves allergy symptoms
Manganum metallicum 8X Relieves hoarseness
Nux vomica 4X Relieves sneezing, coughing, blocked nose
Sabadilla 6X Relieves sneezing, coughing, blocked nose
Sulphur 30X Relieves inflamed nose and eye mucosa
- DIRECTIONS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA HAY FEVER RELIEF
strychnos nux vomica seed - histamine dihydrochloride - manganese - schoenocaulon officinale seed - apis mellifera - arsenic trioxide - silver nitrate - onion - euphrasia stricta - galphimia glauca flowering top - sulfur - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-476 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 4 [hp_X] in 4 g HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 200 [hp_X] in 4 g MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 8 [hp_X] in 4 g SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 4 g ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 30 [hp_X] in 4 g APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 200 [hp_X] in 4 g SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 6 [hp_X] in 4 g EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 5 [hp_X] in 4 g GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 4 [hp_X] in 4 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_X] in 4 g ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 4 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-476-20 2 in 1 BOX 02/17/2021 1 4 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:17089-476-21 1 in 1 BOX 12/15/2021 2 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/17/2021 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-476)