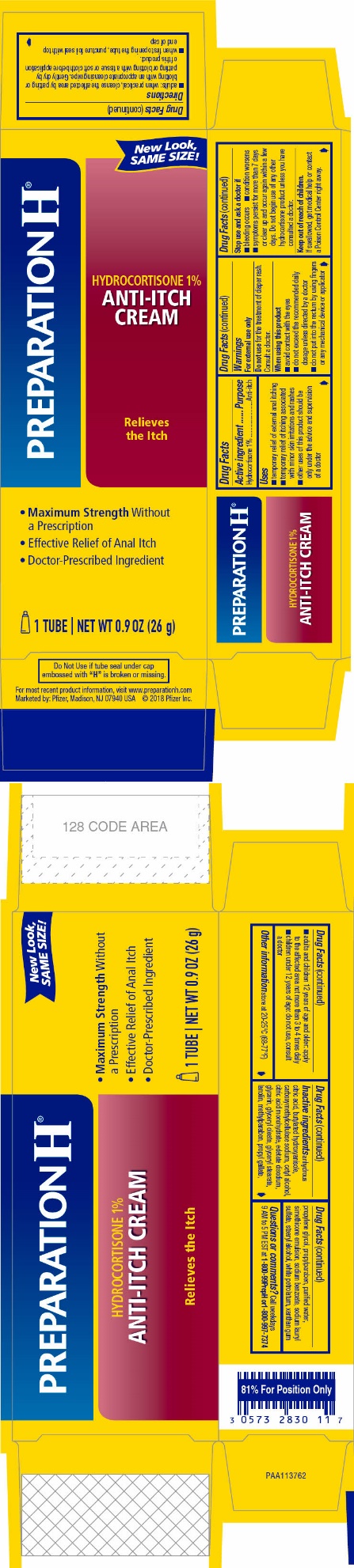
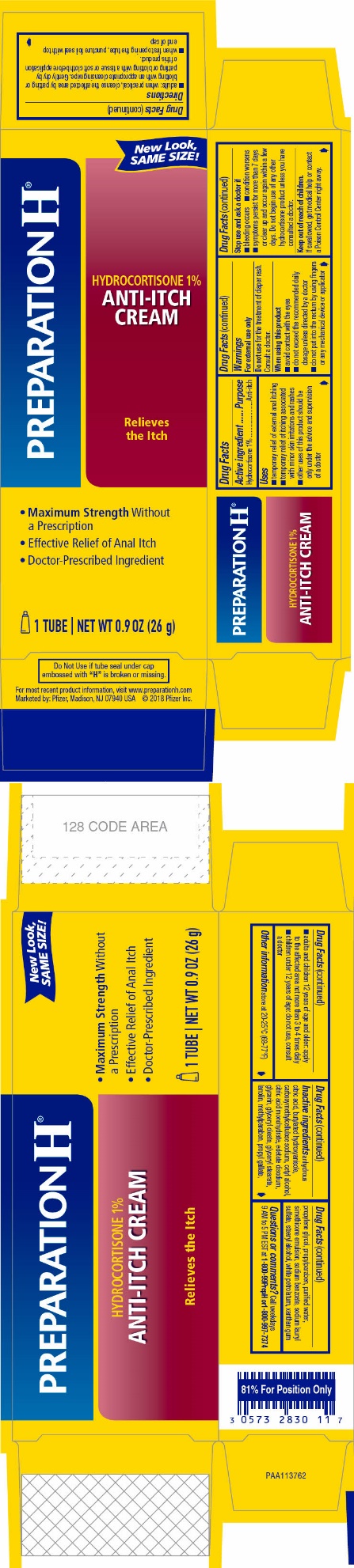
Label: PREPARATION H HYDROCORTISONE- hydrocortisone cream
- NDC Code(s): 0573-2830-10
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
For external use only
When using this product
- avoid contact with the eyes
- do not exceed the recommended daily dosage unless directed by a doctor
- do not put into the rectum by using fingers or any mechanical device or applicator
-
DIRECTIONS
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or soft cloth before application of this product.
- when first opening the tube, puncture foil seal with top end of cap
- adults and children 12 years of age and older: apply to the affected area not more than 3 to 4 times daily
- children under 12 years of age: do not use, consult a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
anhydrous citric acid, butylated hydroxyanisole, carboxymethylcellulose sodium, cetyl alcohol, citric acid monohydrate, edetate disodium, glycerin, glyceryl oleate, glyceryl stearate, lanolin, methylparaben, propyl gallate, propylene glycol, propylparaben, purified water, simethicone emulsion, sodium benzoate, sodium lauryl sulfate, stearyl alcohol, white petrolatum, xanthan gum
- QUESTIONS OR COMMENTS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREPARATION H HYDROCORTISONE
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0573-2830 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL OLEATE (UNII: 4PC054V79P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) DIMETHICONE 350 (UNII: 2Y53S6ATLU) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PETROLATUM (UNII: 4T6H12BN9U) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white (off-white viscous cream) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0573-2830-10 1 in 1 CARTON 01/01/2004 1 26 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 01/01/2004 Labeler - Haleon US Holdings LLC (079944263)