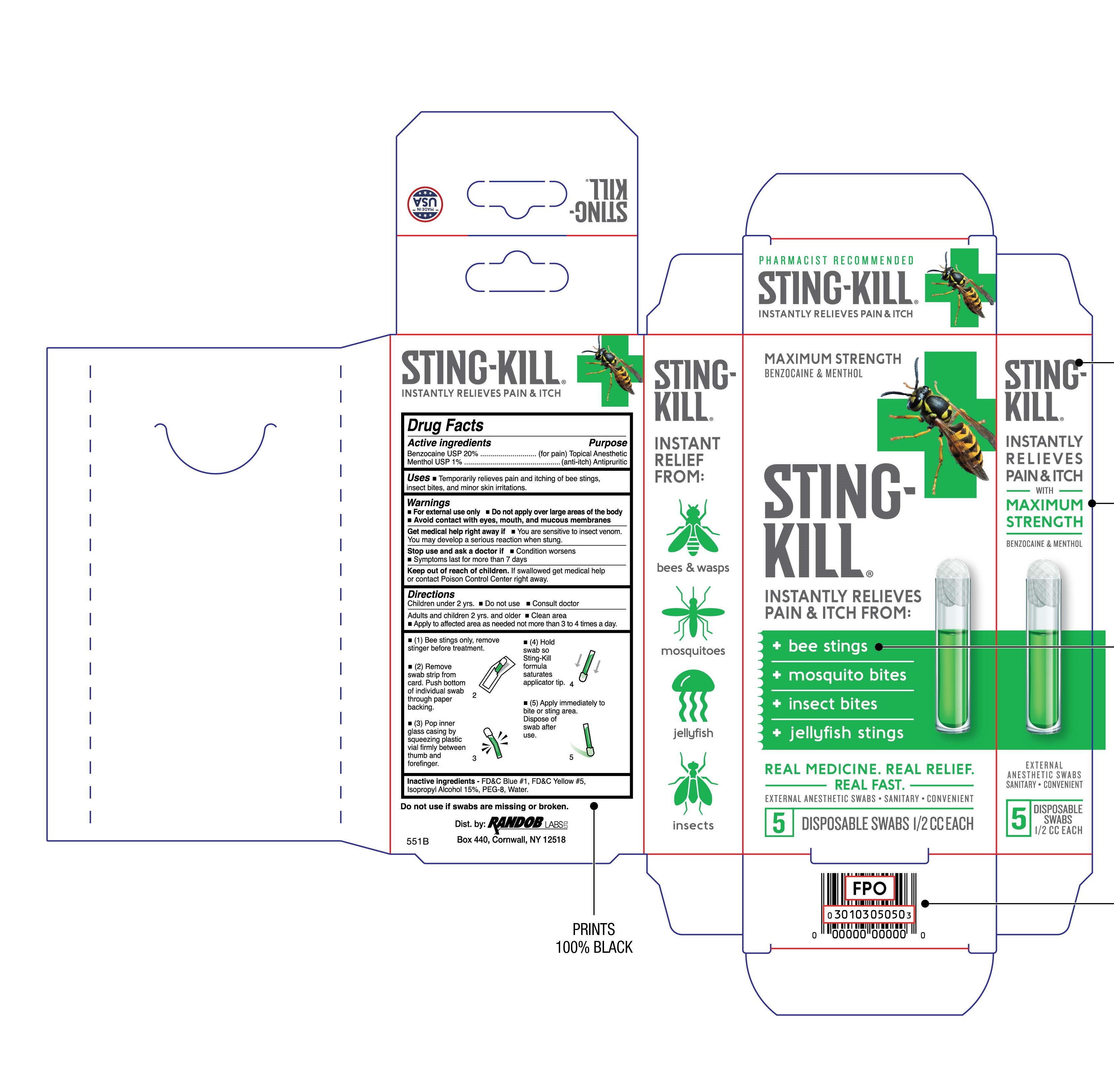
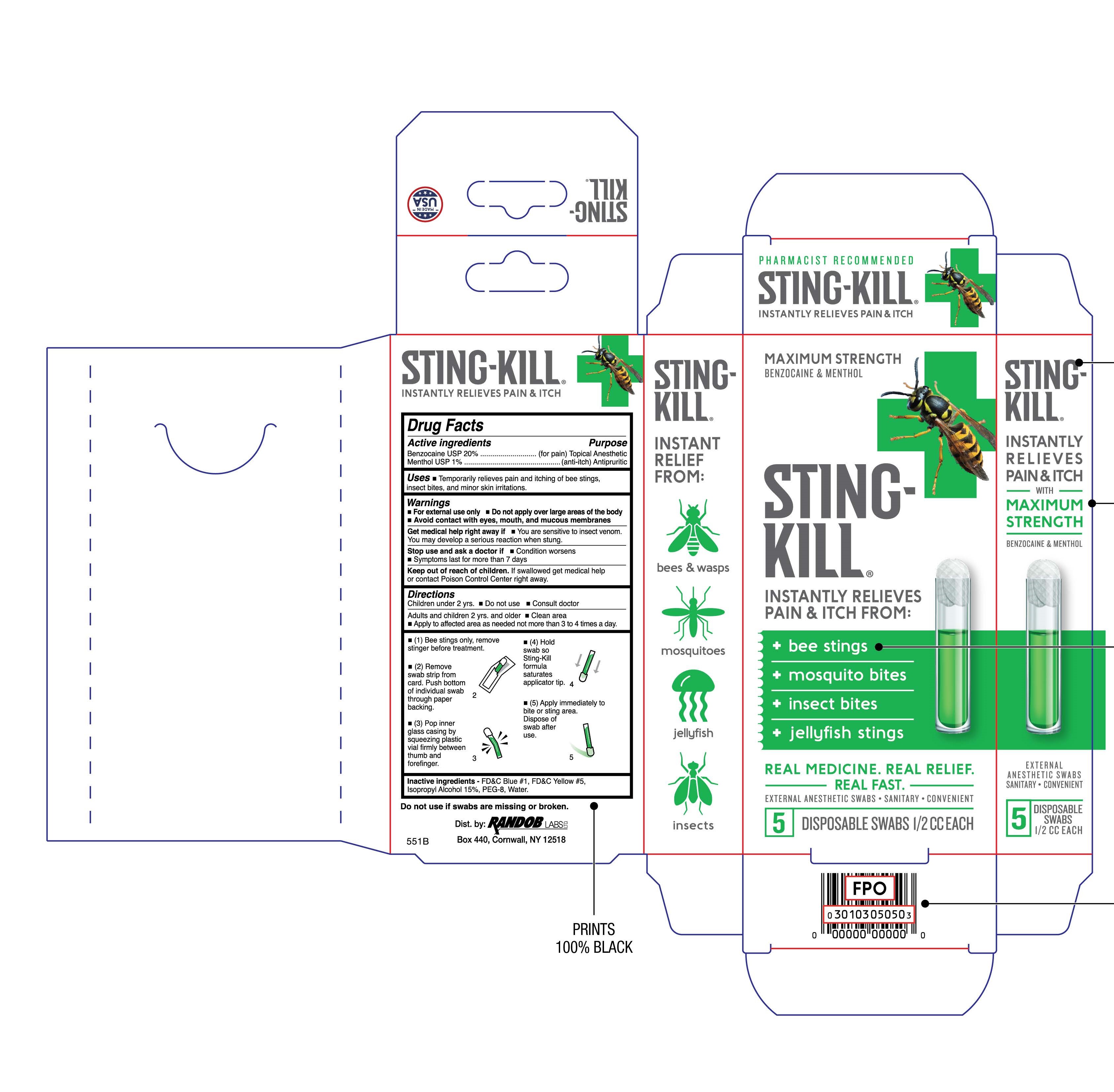
Label: STING-KILL- benzocaine and menthol solution
- NDC Code(s): 52412-200-10, 52412-200-11, 52412-200-12
- Packager: RANDOB LABS, LTD. DBA CROSSINGWELL CONSUMER HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Keep Out of Reach of Children
- Uses
- Warnings
-
Directions
Children under 2 yrs.
- Do not use
- Consult doctor
Adults and children 2 yrs. and older
- Apply to affected area as needed not more than 3 to 4 times a day.
- (1) Bee stings only, remove stinger before treatment.
- (2) Remove strip of swabs from card. Push bottom of individual swab through paper backing.
- (3) Pop inner glass casing by squeezing plastic vial firmly between thumb and forefinger.
- (4) Hold swab so Sting-Kill formula saturates applicator tip.
- (5) Apply immediately to bite or sting area. Dispose of swab after use.
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
STING-KILL
benzocaine and menthol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52412-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ISOPROPYL ALCOHOL (UNII: ND2M416302) PEG-8 LAURATE (UNII: 762O8IWA10) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue (Blue) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52412-200-10 5 in 1 BLISTER PACK 01/01/1965 1 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:52412-200-11 1 in 1 CARTON 01/15/2017 2 5 in 1 BLISTER PACK 2 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC:52412-200-12 2 in 1 CARTON 01/01/1996 3 5 in 1 BLISTER PACK 3 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/1965 Labeler - RANDOB LABS, LTD. DBA CROSSINGWELL CONSUMER HEALTH (061995007) Registrant - RANDOB LABS, LTD. DBA CROSSINGWELL CONSUMER HEALTH (061995007)