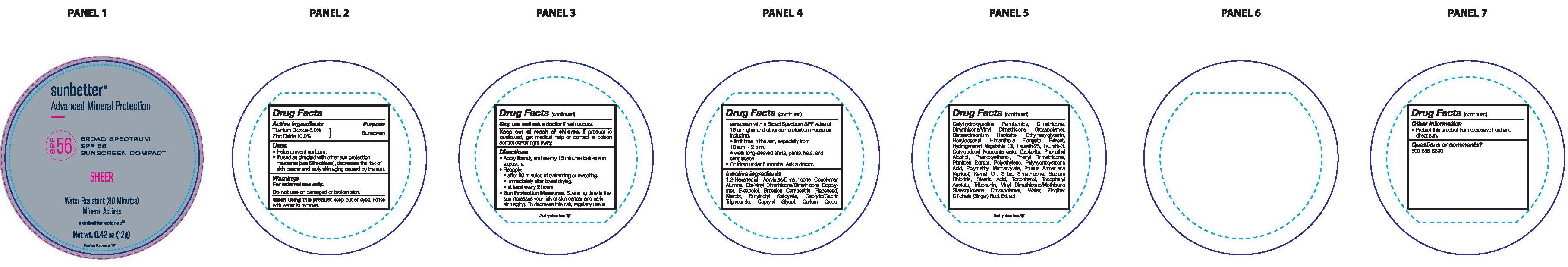
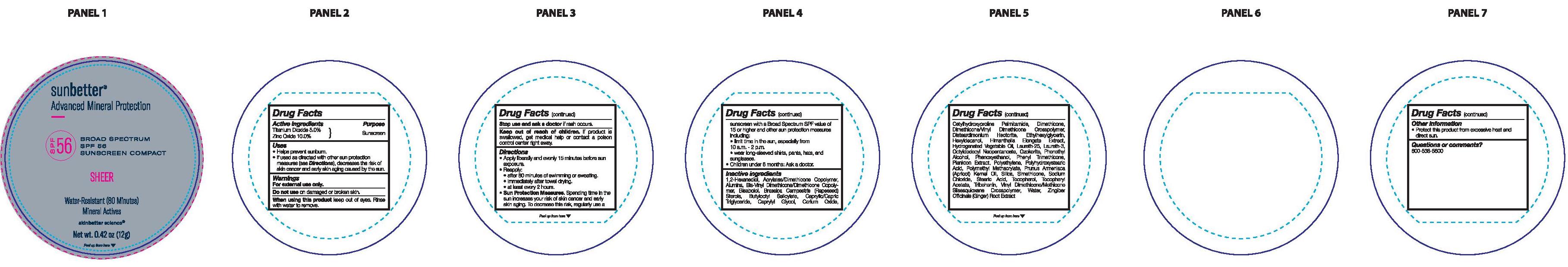
Label: SUNBETTER BROAD SPECTRUM SPF 56 SUNSCREEN COMPACT SHEER- zinc oxide, titanium dioxide stick
- NDC Code(s): 73291-0005-1
- Packager: SKINBETTER SCIENCE LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Sun Protection Measures. Spending time in the sun ircreases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months: Ask a doctor.
-
Inactive ingredients
1,2-Hexanediol, Acrylates/Dimethicone Copolymer, Alumina, Bis-Vinyl Dimethicone/Dimethicone Copolymer, Bisabolol, Brassica Campestris (Rapeseed) Sterols, Butyloctyl Salicylate, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cerium Oxide, Cetylhydroxyproline Palmitamide, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Disteardimonium Hectorite, Ethylhexylglycerin, Hexyldecanol, Himanthalia Elongata Extract, Hydrogenated Vegetable Oil, Laureth-25, Laureth-3, Octyldodecyl Neopentanoate, Ozokerite, Phenethyl Alcohol, Phenoxyethanol, Phenyl Trimethicone, Plankton Extract, Polyethylene, Polyhydroxystearic Acid, Polymethyl Methacrylate, Prunus Armeniaca (Apricot) Kernel Oil, Silica, Simethicone, Sodium Chloride, Stearic Acid, Tocopherol, Tocopheryl Acetate, Tribehenin, Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer, Water, Zingiber Officinale (Ginger) Root Extract.
- Other information
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SUNBETTER BROAD SPECTRUM SPF 56 SUNSCREEN COMPACT SHEER
zinc oxide, titanium dioxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73291-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 mg in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) RAPESEED STEROL (UNII: B46B6DD20U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYLHYDROXYPROLINE PALMITAMIDE (UNII: 74ONU0S62G) LAURETH-25 (UNII: RPD53041LR) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIMETHICONE METHACRYLATE (3000 MW) (UNII: LO1KC6D3D5) HIMANTHALIA ELONGATA (UNII: 21RND18XRR) CORN OIL (UNII: 8470G57WFM) LAURETH-3 (UNII: F32E4CB0UJ) CERESIN (UNII: Q1LS2UJO3A) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) APRICOT KERNEL OIL (UNII: 54JB35T06A) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIBEHENIN (UNII: 8OC9U7TQZ0) WATER (UNII: 059QF0KO0R) GINGER (UNII: C5529G5JPQ) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERIC OXIDE (UNII: 619G5K328Y) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYLDECANOL (UNII: 151Z7P1317) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73291-0005-1 12 g in 1 CONTAINER; Type 0: Not a Combination Product 02/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/01/2021 Labeler - SKINBETTER SCIENCE LLC (049403602)