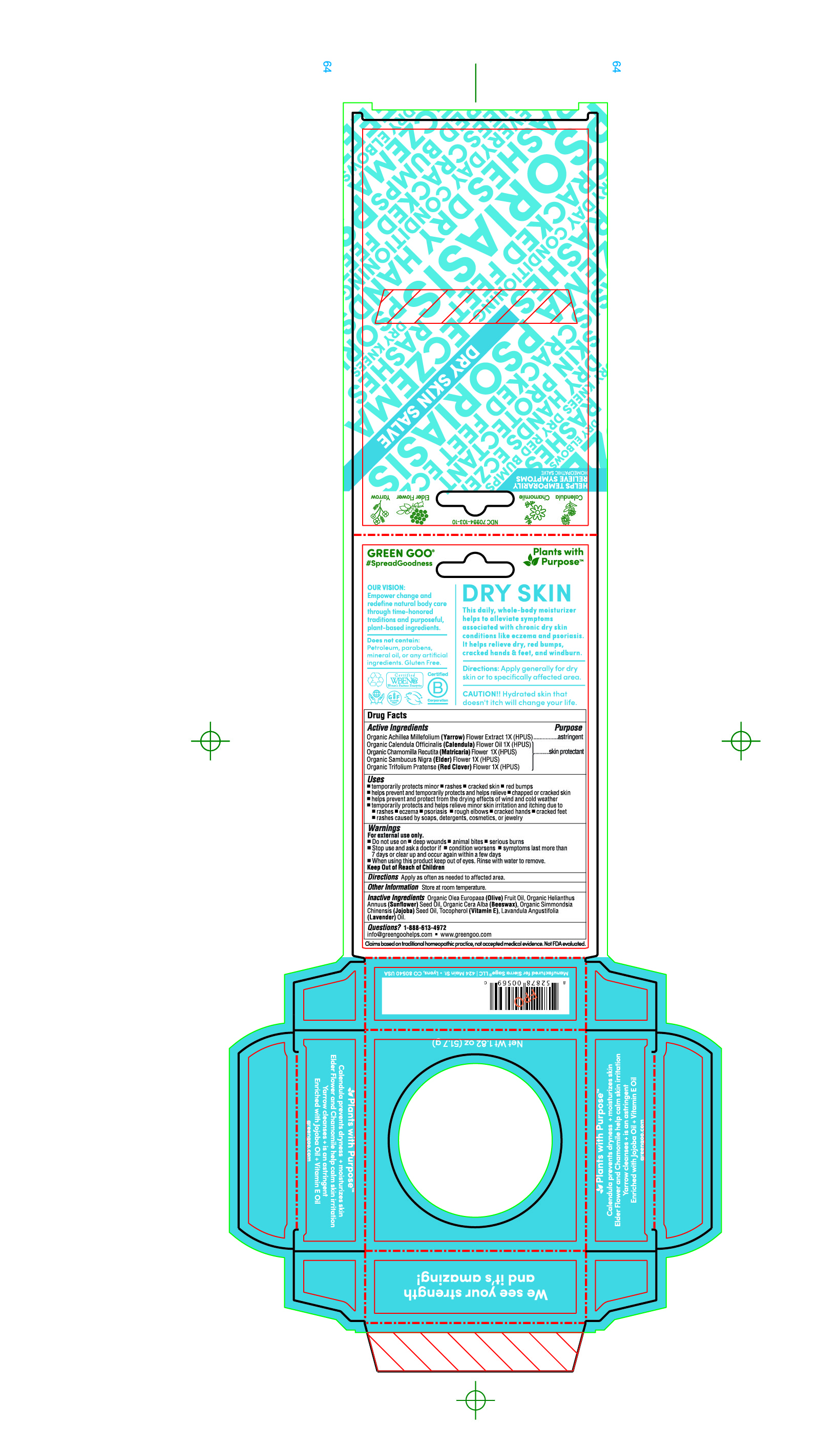
Label: DRY SKIN SALVE- calendula officials flowers, chamollia recutita flowers, viola tricolor leaf, sambucus nigra flowers, trifolium pratense flowers, archillea millefolium salve
-
NDC Code(s):
70994-111-01,
70994-111-02,
70994-111-04,
70994-111-08, view more70994-111-09, 70994-111-10
- Packager: Sierra Sage Herbs LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose Section
- Active Ingredients
-
Indications & Usage
■ temporarily protects minor ■ rashes ■ cracked skin ■ red bumps
■ helps prevent and temporarily protects and helps relieve ■ chapped or cracked skin■ helps prevent and protect from the drying effects of wind and cold weather
■ temporarily protects and helps relieve minor skin irritation and itching due to■ rashes ■ eczema ■ psoriasis ■ rough elbows ■ cracked hands ■ cracked feet
■ rashes caused by soaps, detergents, cosmetics, or jewelry
- Keep out of reach of children section
- Warnings Section
- Storage
- Dosage & Administration Section
- Inactive Ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DRY SKIN SALVE
calendula officials flowers, chamollia recutita flowers, viola tricolor leaf, sambucus nigra flowers, trifolium pratense flowers, archillea millefolium salveProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70994-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 51.7 g CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 1 [hp_X] in 51.7 g TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 1 [hp_X] in 51.7 g SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) (SAMBUCUS NIGRA FLOWER - UNII:07V4DX094T) SAMBUCUS NIGRA FLOWER 1 [hp_X] in 51.7 g ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 1 [hp_X] in 51.7 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) LAVENDER OIL (UNII: ZBP1YXW0H8) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) JOJOBA OIL (UNII: 724GKU717M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70994-111-04 17 g in 1 TUBE; Type 0: Not a Combination Product 04/01/2019 2 NDC:70994-111-09 1 in 1 BOX 04/01/2019 2 NDC:70994-111-01 19.8 g in 1 CAN; Type 0: Not a Combination Product 3 NDC:70994-111-10 1 in 1 BOX 04/01/2019 3 NDC:70994-111-02 51.7 g in 1 CAN; Type 0: Not a Combination Product 4 NDC:70994-111-08 113.4 g in 1 JAR; Type 0: Not a Combination Product 04/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/01/2019 Labeler - Sierra Sage Herbs LLC (006970452)