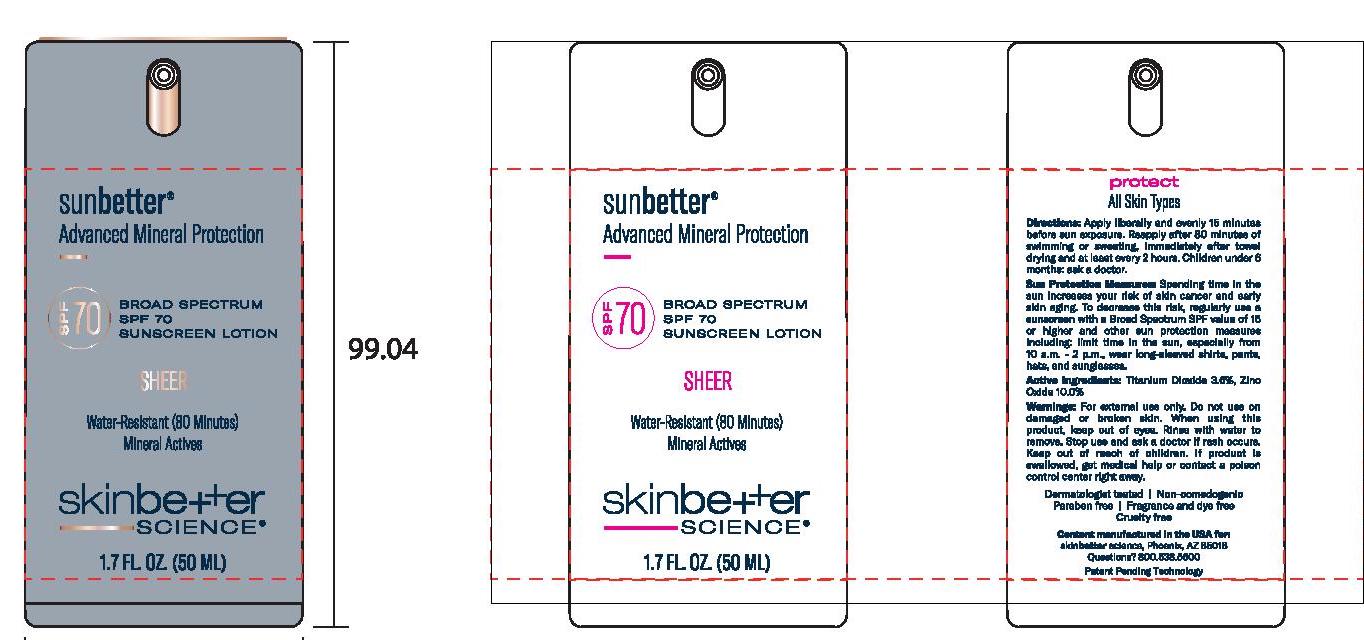
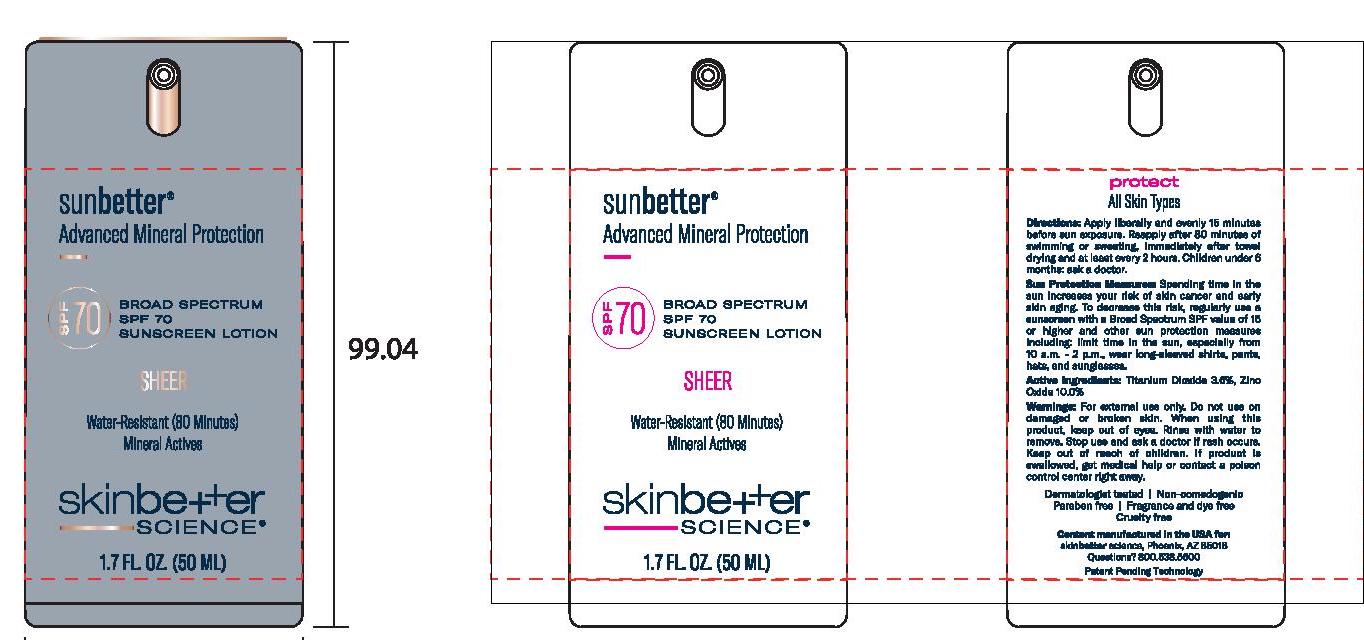
Label: SUNBETTER BROAD SPECTRUM SPF 70 SUNSCREEN SHEER- zinc oxide, titanium dioxide lotion
- NDC Code(s): 73291-0004-1, 73291-0004-2, 73291-0004-3
- Packager: SKINBETTER SCIENCE LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats and sunglasses.
Children under 6 months of age: Ask a doctor.
-
Inactive ingredients
1,2-Hexanediol, Acrylates/Dimethicone Copolymer, Allantoin, Alumina, Bis-Vinyl Dimethicone/Dimethicone Copolymer, Bisabolol, Butyloctyl Salicylate, Camellia Oleifera Leaf Extract, Capparis Spinosa Fruit Extract, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cerium Oxide, Cetyl Dimethicone, Dimethicone, Disteardimonium Hectorite, Glycerin, Himanthalia Elongata Extract, Niacinamide, Olea Europaea (Olive) Fruit Extract, Olea Europaea (Olive) Leaf Extract, Opuntia Ficus-Indica Stem Extract, Panthenol, Phenethyl Alcohol, Phenoxyethanol, Phenyl Trimethicone, Plankton Extract, Polyglyceryl-3 Sorbityl Linseedate, Polyglyceryl-4 Oleate, Polygonum Aviculare Extract, Polyhydroxystearic Acid, Polymethyl Methacrylate, Potassium Sorbate, Silica, Sodium Benzoate, Sodium Chloride, Squalane, Stearic Acid, Styrene/Acrylates Copolymer, Synthetic Fluorphlogopite, Tocopheryl Acetate, Triethyl Citrate, Tropolone, Ubiquinone, Water, Zea Mays (Corn) Starch, Zingiber Officinale (Ginger) Root Extract.
- Other information
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SUNBETTER BROAD SPECTRUM SPF 70 SUNSCREEN SHEER
zinc oxide, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73291-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYGLYCERYL-4 OLEATE (UNII: 15B05TY4GX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) HIMANTHALIA ELONGATA (UNII: 21RND18XRR) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) CERIC OXIDE (UNII: 619G5K328Y) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL DIMETHICONE 45 (UNII: IK315POC44) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) DODECAMETHYLPENTASILOXANE (UNII: 0QDQ2VQ5YJ) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SQUALANE (UNII: GW89575KF9) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73291-0004-1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2021 2 NDC:73291-0004-2 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2021 3 NDC:73291-0004-3 1 mL in 1 PACKET; Type 0: Not a Combination Product 02/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/01/2021 Labeler - SKINBETTER SCIENCE LLC (049403602)