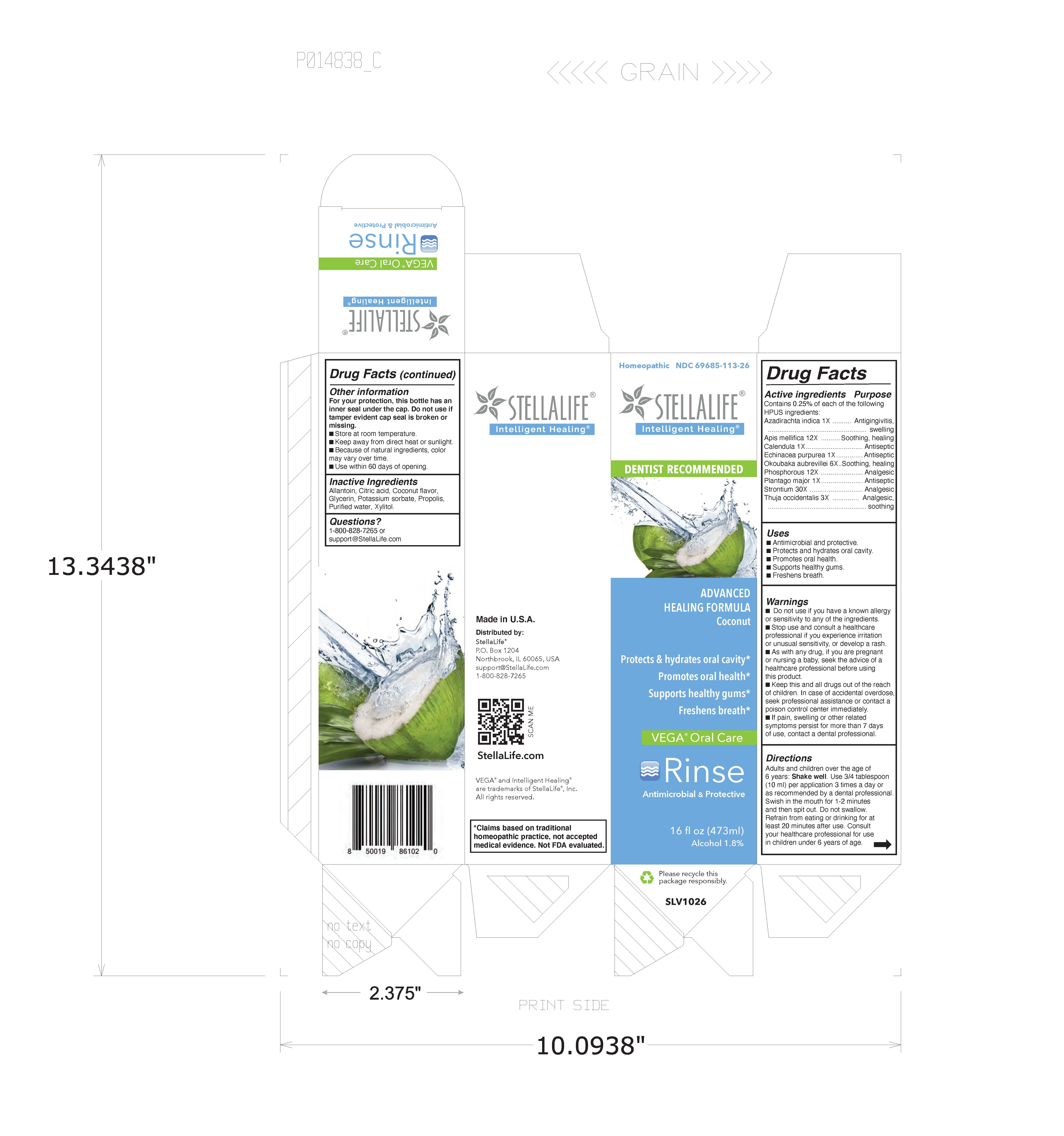
Label: STELLALIFE VEGA ORAL CARE COCONUT- risne rinse
- NDC Code(s): 69685-113-16
- Packager: StellaLife, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 26, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Contains 0.25% of each of the following HPUS ingredients:
Active Ingredients Purpose Azadirachta indica 1X Antigingivitis, swelling Apis mellifica 12X Soothing, healing Calendula 1X Antiseptic Echinacea purpurea 1X Antiseptic Okoubaka aubrevellei 6X Soothing, healing Phosphorous 12X Anaglesic Plantago major 1X Antiseptic Strontium 30X Analgesic Thuja occidentalis 3X Analgesic, soothing - Uses
- Usage
-
Warnings
- Do not use if you have a known allergy or sensitivity to any of the following ingredients
- Stop use and consult a healthcare professional if you experience irritation or unusual sensitivity, or develop a rash.
- As with any drug, if you are pregnant or nursing a baby, seek the advise of a healthcare professional before using this product.
- Keep this and all drugs out of the reach of children. In case of accidental overdose, seek professional assistance or contact poison control center immediately.
- If pain, swelling or other related symptoms persist for more than 7 days of use, contact a dental professional.
- Keep Out of Reach of Children
-
Directions
Adults and children ovver the age of 6 years: Shake well. Use 3/4 tablespoon (10 ml) per application 3 times a day. Swish in the mouth for 1-2 minutes abnd then spit out. Do not swallow. Refrain from eating or drinking for at least 20 minutes after use. Consult your healthcare professional for use in children under 6 years of age.
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STELLALIFE VEGA ORAL CARE COCONUT
risne rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69685-113 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 1 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 12 [hp_X] in 1 mL AZADIRACHTA INDICA BARK (UNII: G580B439YI) (AZADIRACHTA INDICA BARK - UNII:G580B439YI) AZADIRACHTA INDICA BARK 1 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 3 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 1 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 1 [hp_X] in 1 mL STRONTIUM (UNII: YZS2RPE8LE) (STRONTIUM - UNII:YZS2RPE8LE) STRONTIUM 30 [hp_X] in 1 mL OKOUBAKA AUBREVILLEI BARK (UNII: MK2074187Z) (OKOUBAKA AUBREVILLEI BARK - UNII:MK2074187Z) OKOUBAKA AUBREVILLEI BARK 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) PROPOLIS WAX (UNII: 6Y8XYV2NOF) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) COCONUT (UNII: 3RT3536DHY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALLANTOIN (UNII: 344S277G0Z) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color yellow Score Shape Size Flavor COCONUT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69685-113-16 1 in 1 BOX 01/08/2021 1 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/08/2021 Labeler - StellaLife, Inc. (079714251) Registrant - Homeocare Laboratories, Inc. (088248828) Establishment Name Address ID/FEI Business Operations Homeocare Laboratories Inc 088248828 manufacture(69685-113)