Label: ADULT GLYCERIN LAXATIVE- glycerin suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 49781-081-25 - Packager: Cardinal Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 19, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active ingredient
- Purpose
- Keep out of reach of children.
- Use
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor
- If pregnant or breastfeeding,
-
Directions
Single Daily dosage: 1 suppository or as directed by a doctor
- Adults and children 6 years and over:
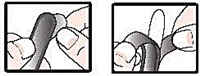
- detach 1 suppository from the strip; remove the wrapper before inserting into the rectum as follows:
- hold suppository with pointed end up
- carefully separate tabs with fingernail
- slowly pull apart by pulling tabs down on both sides
- remove suppository from wrapper
- insert one suppository well up into the rectum
- children 2 to 6 years use Leader Pediatric Glycerin Suppositories
- children under 2 years of age ask a doctor
- Inactive ingredients
- Other information
-
Adult Glycerin Suppositories Label
NDC 49718-081-25
Leader®
Compare to Fleet® active ingredient*
Glycerin Suppositories
LaxativeSATISFACTION GUARANTEED
25 Adult Size
TAMPER-EVIDENT FOR YOUR SAFETY, SUPPOSITORIES ARE PACKED IN TAMPER-EVIDENT SEALED WRAPPER. DO NOT USE IF WRAPPER IS TORN OR OPEN
E-Z Open Wrapper
Distributed by Cardinal Health, Inc.
Dublin, OH 43017 CIN # 4866216
www.myleader.com 1-800-200-6313ALL LEADER® Brand Products are 100% satisfaction guaranteed or return to place of purchase for a full refund.
This product is not manufactured or distributed by C.B. Fleet Co. Inc., owner of the registered trademark Fleet®

-
INGREDIENTS AND APPEARANCE
ADULT GLYCERIN LAXATIVE
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49781-081 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g Inactive Ingredients Ingredient Name Strength SODIUM STEARATE (UNII: QU7E2XA9TG) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49781-081-25 25 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/01/2014 Labeler - Cardinal Health, Inc. (097537435) Registrant - Acino Products LLC (019385518) Establishment Name Address ID/FEI Business Operations Acino Products LLC 019385518 manufacture(49781-081)

