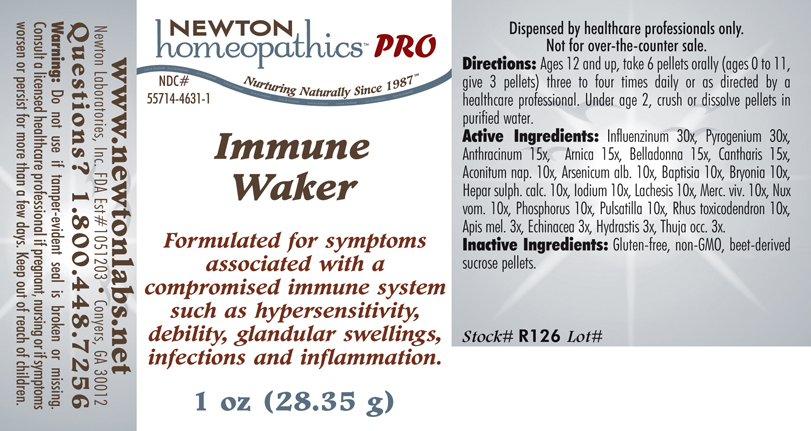
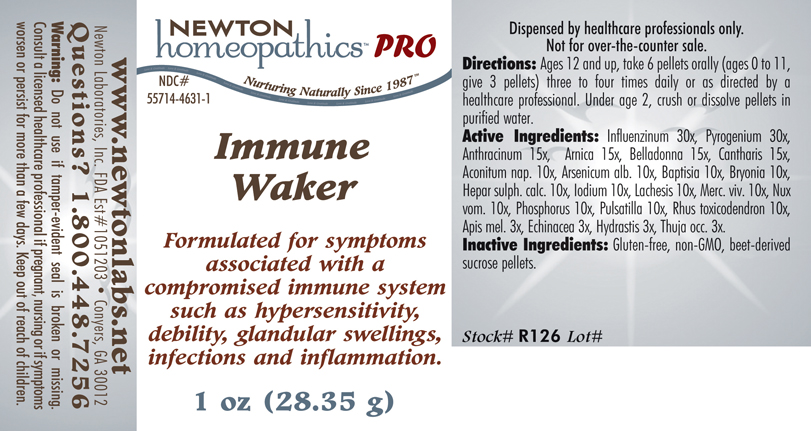
Label: IMMUNE WAKER- influenzinum, pyrogenium, anthracinum,arnica, belladonna, cantharis, aconitum nap., arsenicum alb., baptisia, bryonia, hepar sulph. calc., iodium, lachesis, merc. viv., nux vom., phosphorus, pulsatilla, rhus toxicodendron, apis mel., echinacea, hydrastis, thuja occ. plaster
-
Contains inactivated NDC Code(s)
NDC Code(s): 55714-4631-1, 55714-4631-2 - Packager: Newton Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 30, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE SECTION
- DOSAGE & ADMINISTRATION SECTION
-
ACTIVE INGREDIENT SECTION
Influenzinum 30x, Pyrogenium 30x, Anthracinum 15x, Arnica 15x, Belladonna 15x, Cantharis 15x, Aconitum nap. 10x, Arsenicum alb.10x, Baptisia 10x, Bryonia 10x, Hepar sulph. calc. 10x, Iodium 10x, Lachesis 10x, Merc. viv. 10x, Nux vom. 10x, Phosphorus 10x, Pulsatilla 10x, Rhus toxicodendron 10x, Apis mel. 3x, Echinacea 3x, Hydrastis 3x, Thuja occ. 3x.
- PURPOSE SECTION
- INACTIVE INGREDIENT SECTION
-
QUESTIONS SECTION
www.newtonlabs.net Newton Laboratories, Inc. FDA Est # 1051203 - Conyers, GA 30012 Questions? 1.800.448.7256
- WARNINGS SECTION
- PREGNANCY OR BREAST FEEDING SECTION
- KEEP OUT OF REACH OF CHILDREN SECTION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
IMMUNE WAKER
influenzinum, pyrogenium, anthracinum,arnica, belladonna, cantharis, aconitum nap., arsenicum alb., baptisia, bryonia, hepar sulph. calc., iodium, lachesis, merc. viv., nux vom., phosphorus, pulsatilla, rhus toxicodendron, apis mel., echinacea, hydrastis, thuja occ. plasterProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55714-4631 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Influenza A Virus (UNII: R9HH0NDE2E) (Influenza A Virus - UNII:R9HH0NDE2E) Influenza A Virus 30 [hp_X] in 1 g Rancid Beef (UNII: 29SUH5R3HU) (Rancid Beef - UNII:29SUH5R3HU) Rancid Beef 30 [hp_X] in 1 g Bacillus Anthracis Immunoserum Rabbit (UNII: 41LZ22DD4N) (Bacillus Anthracis Immunoserum Rabbit - UNII:41LZ22DD4N) Bacillus Anthracis Immunoserum Rabbit 15 [hp_X] in 1 g Arnica Montana (UNII: O80TY208ZW) (Arnica Montana - UNII:O80TY208ZW) Arnica Montana 15 [hp_X] in 1 g Atropa Belladonna (UNII: WQZ3G9PF0H) (Atropa Belladonna - UNII:WQZ3G9PF0H) Atropa Belladonna 15 [hp_X] in 1 g Lytta Vesicatoria (UNII: 3Q034RO3BT) (Lytta Vesicatoria - UNII:3Q034RO3BT) Lytta Vesicatoria 15 [hp_X] in 1 g Aconitum Napellus (UNII: U0NQ8555JD) (Aconitum Napellus - UNII:U0NQ8555JD) Aconitum Napellus 10 [hp_X] in 1 g Arsenic Trioxide (UNII: S7V92P67HO) (Arsenic Cation (3+) - UNII:C96613F5AV) Arsenic Trioxide 10 [hp_X] in 1 g Baptisia Tinctoria Root (UNII: 5EF0HWI5WU) (Baptisia Tinctoria Root - UNII:5EF0HWI5WU) Baptisia Tinctoria Root 10 [hp_X] in 1 g Bryonia Alba Root (UNII: T7J046YI2B) (Bryonia Alba Root - UNII:T7J046YI2B) Bryonia Alba Root 10 [hp_X] in 1 g Calcium Sulfide (UNII: 1MBW07J51Q) (Calcium Sulfide - UNII:1MBW07J51Q) Calcium Sulfide 10 [hp_X] in 1 g Iodine (UNII: 9679TC07X4) (Iodine - UNII:9679TC07X4) Iodine 10 [hp_X] in 1 g Lachesis Muta Venom (UNII: VSW71SS07I) (Lachesis Muta Venom - UNII:VSW71SS07I) Lachesis Muta Venom 10 [hp_X] in 1 g Mercury (UNII: FXS1BY2PGL) (Mercury - UNII:FXS1BY2PGL) Mercury 10 [hp_X] in 1 g Strychnos Nux-vomica Seed (UNII: 269XH13919) (Strychnos Nux-vomica Seed - UNII:269XH13919) Strychnos Nux-vomica Seed 10 [hp_X] in 1 g Phosphorus (UNII: 27YLU75U4W) (Phosphorus - UNII:27YLU75U4W) Phosphorus 10 [hp_X] in 1 g Pulsatilla Vulgaris (UNII: I76KB35JEV) (Pulsatilla Vulgaris - UNII:I76KB35JEV) Pulsatilla Vulgaris 10 [hp_X] in 1 g Toxicodendron Pubescens Leaf (UNII: 6IO182RP7A) (Toxicodendron Pubescens Leaf - UNII:6IO182RP7A) Toxicodendron Pubescens Leaf 10 [hp_X] in 1 g Apis Mellifera (UNII: 7S82P3R43Z) (Apis Mellifera - UNII:7S82P3R43Z) Apis Mellifera 3 [hp_X] in 1 g Echinacea, Unspecified (UNII: 4N9P6CC1DX) (Echinacea, Unspecified - UNII:4N9P6CC1DX) Echinacea, Unspecified 3 [hp_X] in 1 g Goldenseal (UNII: ZW3Z11D0JV) (Goldenseal - UNII:ZW3Z11D0JV) Goldenseal 3 [hp_X] in 1 g Thuja Occidentalis Leafy Twig (UNII: 1NT28V9397) (Thuja Occidentalis Leafy Twig - UNII:1NT28V9397) Thuja Occidentalis Leafy Twig 3 [hp_X] in 1 g Influenza B Virus (UNII: 1314JZ2X6W) (Influenza B Virus - UNII:1314JZ2X6W) Influenza B Virus 30 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55714-4631-1 28.35 g in 1 BOTTLE, GLASS 2 NDC:55714-4631-2 56.7 g in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/30/2013 Labeler - Newton Laboratories, Inc. (788793610) Registrant - Newton Laboratories, Inc. (788793610) Establishment Name Address ID/FEI Business Operations Newton Laboratories, Inc. 788793610 MANUFACTURE(55714-4631)