Label: ALLEROFF- cetirizine hydrochloride syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 16853-1261-1, 16853-1261-2 - Packager: Corporacion Infarmasa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 15, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- STOP USE
- PURPOSE
- QUESTIONS
- STORAGE AND HANDLING
-
DOSAGE & ADMINISTRATION
Use only with enclosed dosing cup
Adults and Children 6 years and over 1 teaspoonful (5ml) ot 2 teaspoonful (10ml) once daily depending upon severity of symptoms; do not take more than 2 teaspoonful (10ml) in 24 hours
Adults 65 years and over 1 teaspoonful (5ml) once daily; do not take more than 1 teaspoonful (5ml) in 24 hours
Children 2 to under 6 years of age 1/2 teaspoonful (2.5ml) once daily.If needed, dose can be increased to a maximum of 1 teaspoon (5ml) once daily or 1/2 teaspoonful (2.5ml) every 12 hours. Do not give more than 1 teaspoonful (5ml) in 24 hours
Children under 2 years of age Ask a Doctor
Consumer with liver or kidney disease Ask a Doctor
- INACTIVE INGREDIENT
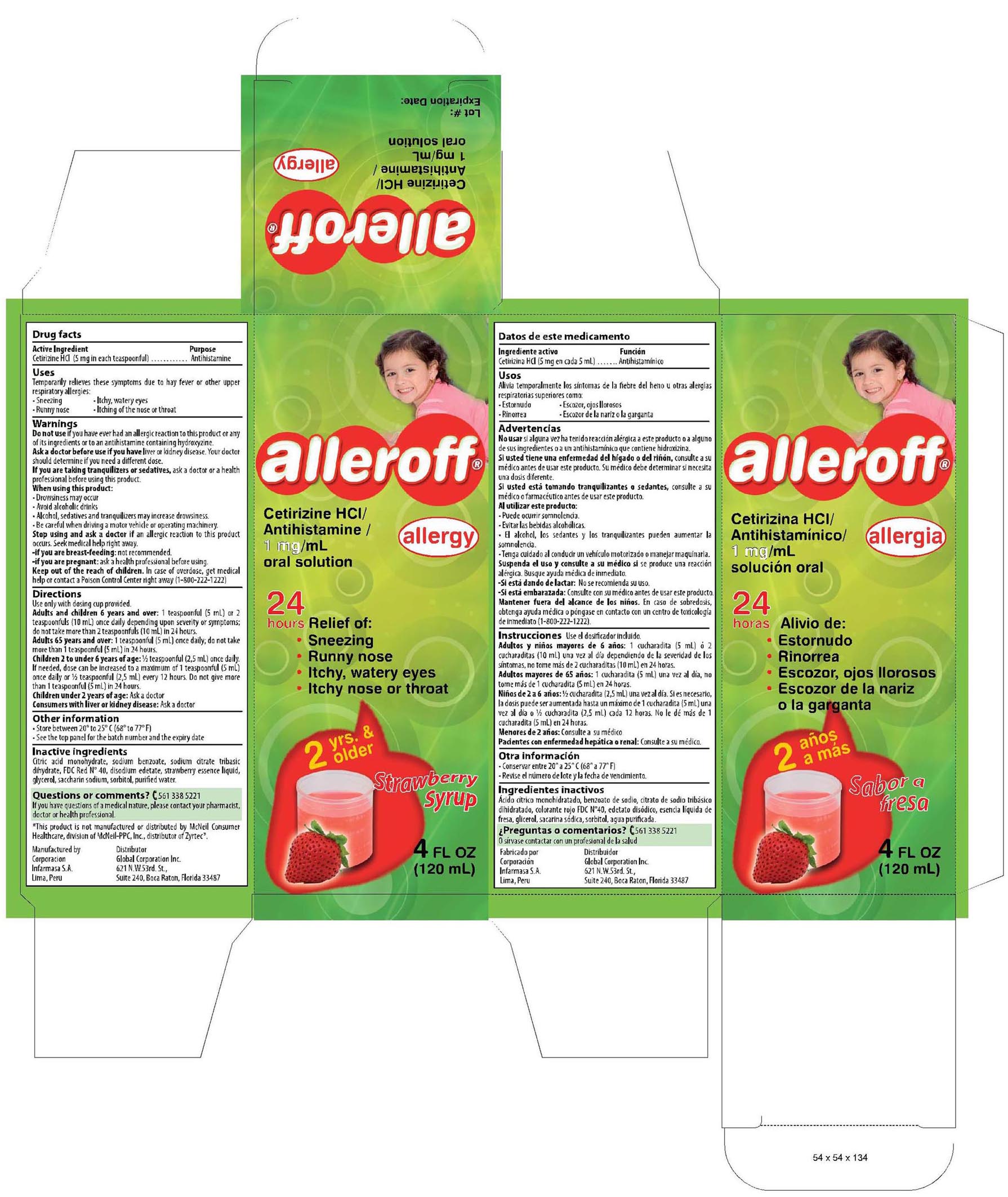
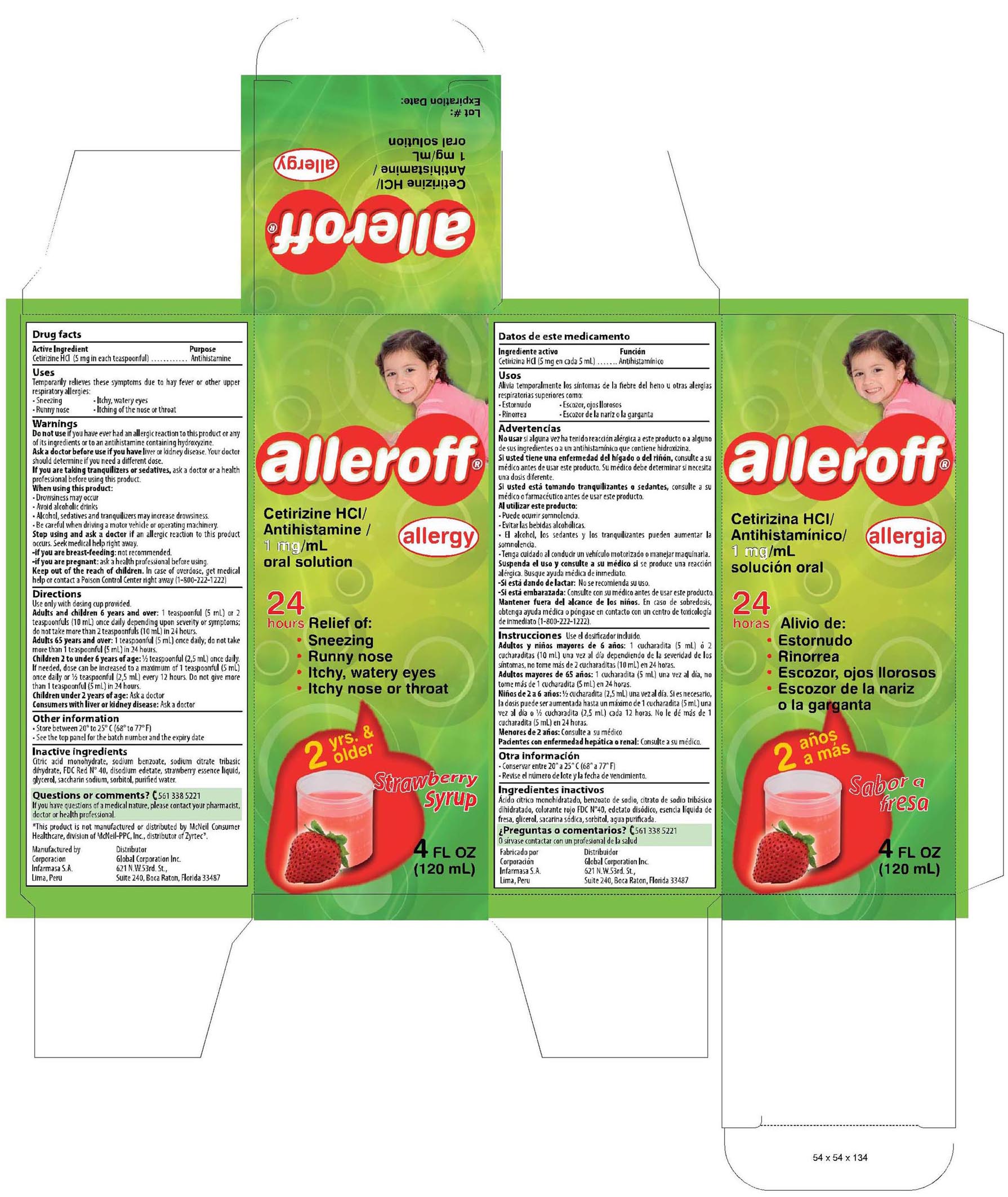
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLEROFF
cetirizine hydrochloride syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16853-1261 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 1 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.080 mg SODIUM BENZOATE (UNII: OJ245FE5EU) 0.100 mg SODIUM CITRATE (UNII: 1Q73Q2JULR) 0.090 mg FD&C RED NO. 40 (UNII: WZB9127XOA) 0.003 mg EDETATE DISODIUM (UNII: 7FLD91C86K) 0.025 mg GLYCERIN (UNII: PDC6A3C0OX) 25 mg SACCHARIN SODIUM (UNII: SB8ZUX40TY) 0.075 mg SORBITOL (UNII: 506T60A25R) 30 mg WATER (UNII: 059QF0KO0R) 100 mL STRAWBERRY (UNII: 4J2TY8Y81V) 0.07 mg Product Characteristics Color pink (pink) Score Shape Size Flavor STRAWBERRY (Strawberry) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16853-1261-1 1 in 1 CARTON 2 NDC:16853-1261-2 2 in 1 PACKAGE, COMBINATION Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022155 01/15/2010 Labeler - Corporacion Infarmasa (934098294) Establishment Name Address ID/FEI Business Operations Corporacion Infarmasa 934098294 manufacture

 Enter section text here
Enter section text here