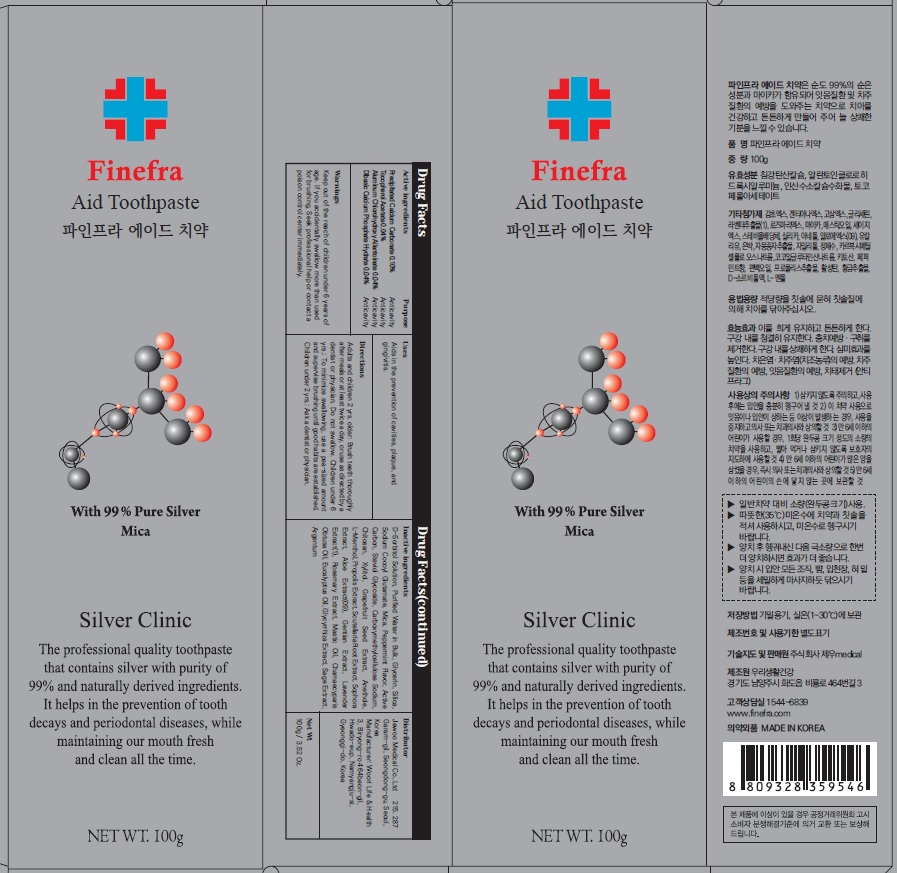
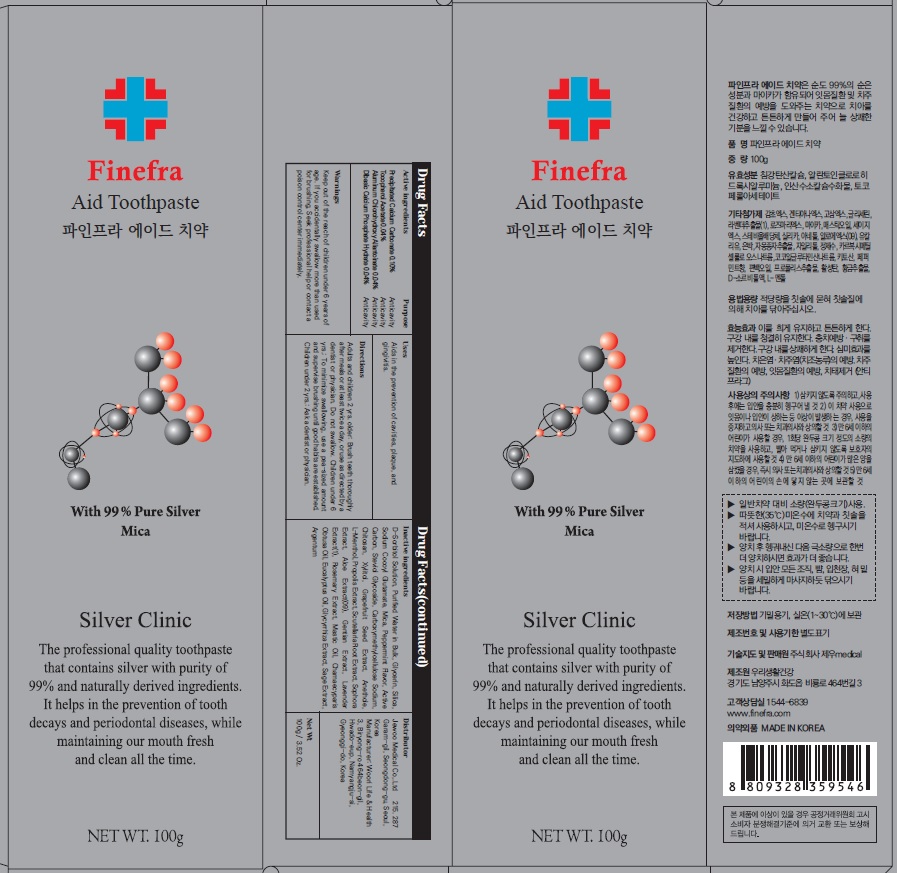
Label: FINEFRA AID TOOTH- precipitated calcium carbonate, tocopherol acetate, aluminum chlorohydroxy allantoinate, dibasic calcium phosphate hydrate paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 69653-130-01, 69653-130-02, 69653-130-03 - Packager: Jewoo Medical Co,.Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 3, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
D-Sorbitol Solution, Purified Water in Bulk, Glycerin, Silica, Sodium Cocoyl Glutamate, Mica, Peppermint Flavor, Active Carbon, Steviol Glycoside, Carboxymethylcellulose Sodium, Chitosan, Xylitol, Grapefruit Seed Extract, Anethole, L-Menthol, Propolis Extract, Scutellaria Root Extract, Sophora Extract, Aloe Extract(09), Gentian Extract, Lavender Extract(1), Rosemary Extract, Mastic Oil, Chamaecyparis Obtusa Oil, Eucalyptus Oil, Glycyrrhiza Extract, Sage Extract, Argentum
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Directions
Directions:
Adults and children 2 yrs. older: Brush teeth thoroughly after meals or at least twice a day, or use as directed by a dentist or physician. Do not swallow. Children under 6 yrs.: To minimize swallowing, use a pea-sized amount and supervise brushing until good habits are established. Children under 2 yrs.: Ask a dentist or physician.
- Package Label: Finefra Aid Toothpaste 20g
- Package Label: Finefra Aid Toothpaste 100g
- Package Label: Finefra Aid Toothpaste 135g
-
INGREDIENTS AND APPEARANCE
FINEFRA AID TOOTH
precipitated calcium carbonate, tocopherol acetate, aluminum chlorohydroxy allantoinate, dibasic calcium phosphate hydrate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69653-130 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 0.10 g in 100 g .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 0.04 g in 100 g ALCLOXA (UNII: 18B8O9DQA2) (ALLANTOIN - UNII:344S277G0Z) ALCLOXA 0.04 g in 100 g DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) DIBASIC CALCIUM PHOSPHATE DIHYDRATE 0.04 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69653-130-01 20 g in 1 TUBE; Type 0: Not a Combination Product 05/01/2020 2 NDC:69653-130-02 100 g in 1 CARTON; Type 0: Not a Combination Product 05/01/2020 3 NDC:69653-130-03 135 g in 1 CARTON; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2020 Labeler - Jewoo Medical Co,.Ltd (689512541) Registrant - Jewoo Medical Co,.Ltd (689512541) Establishment Name Address ID/FEI Business Operations Woorilife&Health 694860803 manufacture(69653-130)