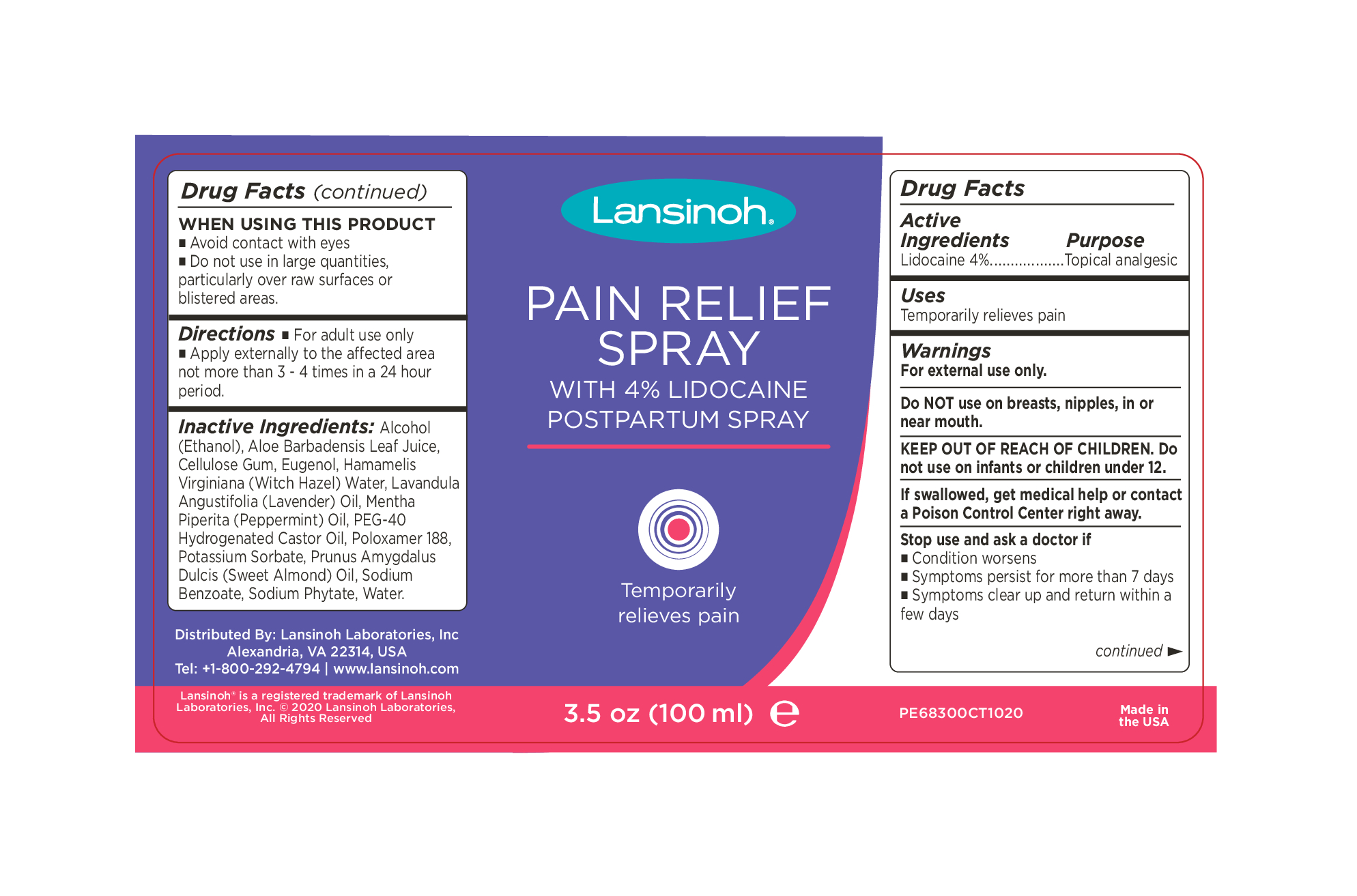
Label: LANSINOH PAIN RELIEVING- lidocaine hcl spray
- NDC Code(s): 53997-001-01, 53997-001-02
- Packager: Lansinoh Laboratories Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- STOP USE AND ASK A DOCTOR
- ASK DOCTOR/PHARMACIST
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- STOP USE
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients:
Alcohol (Ethanol), Aloe Barbadensis Leaf Juice, Cellulose Gum, Eugenol, Hamamelis Virginiana (Witch Hazel) Water, Lavandula Angustifolia (Lavender) Oil, Mentha Piperita (Peppermint) Oil, PEG-40 Hydrogenated Castor Oil, Poloxamer 188, Potassium Sorbate, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Sodium Benzoate, Sodium Phytate, Water.
Distributed By: Lansinoh Laboratories, Inc
Alexandria, VA 22314, USA
Tel: +1-800-292-4794 | www.lansinoh.com - WARNINGS
- INDICATIONS & USAGE
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANSINOH PAIN RELIEVING
lidocaine hcl sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53997-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength PHYTATE SODIUM (UNII: 88496G1ERL) ALOE VERA WHOLE (UNII: KIZ4X2EHYX) PEG-40 CASTOR OIL (UNII: 4ERD2076EF) EUGENOL (UNII: 3T8H1794QW) ALCOHOL (UNII: 3K9958V90M) WITCH HAZEL (UNII: 101I4J0U34) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) POLOXAMER 188 (UNII: LQA7B6G8JG) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ALMOND OIL (UNII: 66YXD4DKO9) PEPPERMINT OIL (UNII: AV092KU4JH) CARBOXYMETHYLCELLULOSE SODIUM (0.7 CARBOXYMETHYL SUBSTITUTION PER SACCHARIDE; 2300 MPA.S AT 1%) (UNII: 0Z2R7OG99L) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53997-001-02 1 in 1 CARTON 02/15/2021 1 NDC:53997-001-01 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/15/2021 Labeler - Lansinoh Laboratories Inc (181696907) Establishment Name Address ID/FEI Business Operations Dhaliwal Pharmaceuticals Laboratories LLC 116933772 manufacture(53997-001)