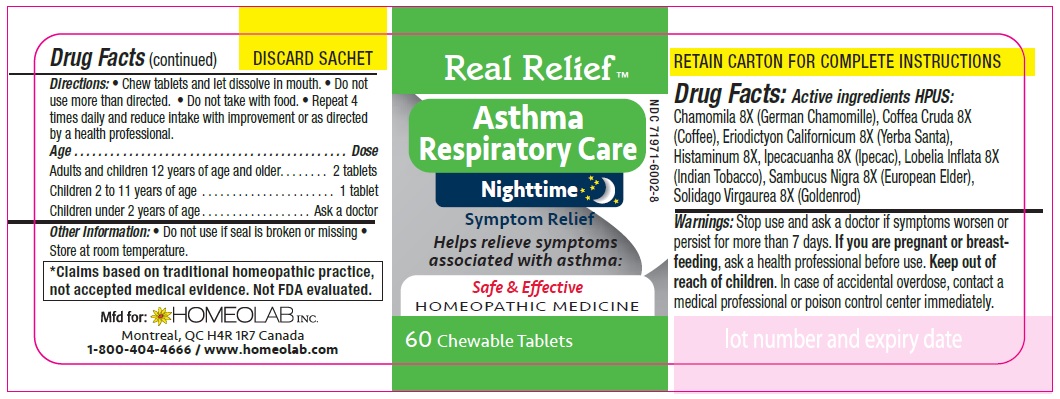
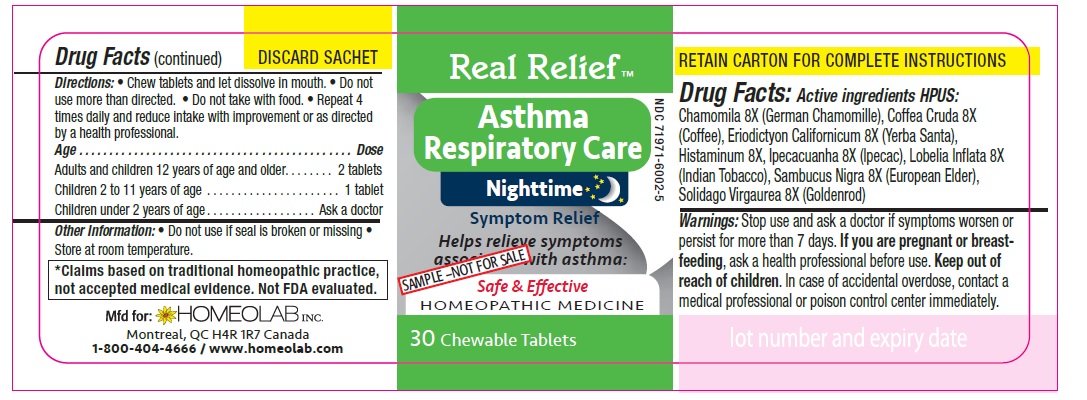
Label: REAL RELIEF- chamomila, coffea cruda, eriodictyon californicum, histaminum, ipecacuanha, lobelia inflata, sambucus nigra, solidago virgaurea tablet
- NDC Code(s): 65808-322-01
- Packager: GMP Laboratories of America, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
Purpose
Homeopathic remedy helps relieve symptoms of asthma:
- gasping for air
- dry cough
- difficulty breathing
- irritability and nocturnal awakenings
- wheezing
- cough with mucus, cough with expectoration
- restlessness and sleeplessness
- excess mucus
- irregular respiration
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 4 times daily and reduce intake with improvement or as directed by a health professional
Age…………………………………………………………Dose
Adults and Children 12 years of age and older………...... 2 tablets
Children 2 to 11 years of age…...……………………............ 1 tablet
Children under 2 years of age……………………................. Ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Product label
-
INGREDIENTS AND APPEARANCE
REAL RELIEF
chamomila, coffea cruda, eriodictyon californicum, histaminum, ipecacuanha, lobelia inflata, sambucus nigra, solidago virgaurea tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65808-322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 8 [hp_X] ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 8 [hp_X] ERIODICTYON CALIFORNICUM LEAF (UNII: 2Y7TIQ135H) (ERIODICTYON CALIFORNICUM LEAF - UNII:2Y7TIQ135H) ERIODICTYON CALIFORNICUM LEAF 8 [hp_X] HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 8 [hp_X] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 8 [hp_X] LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 8 [hp_X] SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 8 [hp_X] SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 8 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code HLB Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65808-322-01 1 in 1 CARTON 01/01/2020 1 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2020 Labeler - GMP Laboratories of America, Inc. (876754375)