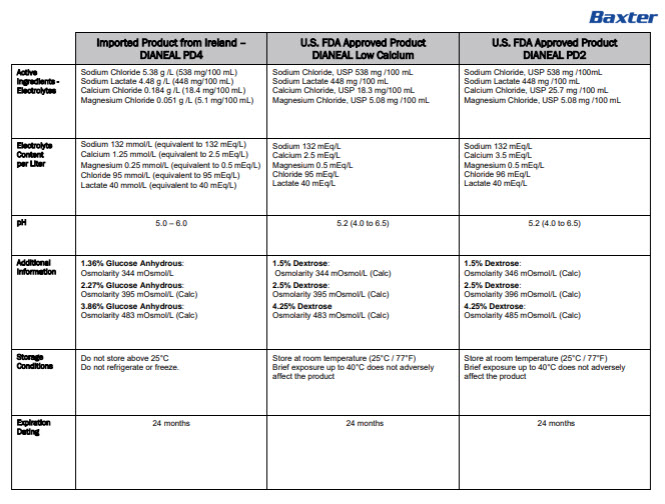
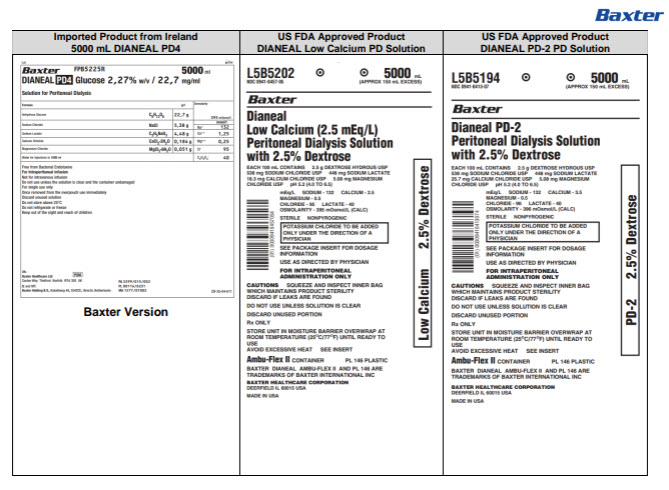
Label: DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
-
NDC Code(s):
0941-0727-01,
0941-0727-02,
0941-0729-01,
0941-0729-02, view more0941-0731-01, 0941-0731-02
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Lot Exp
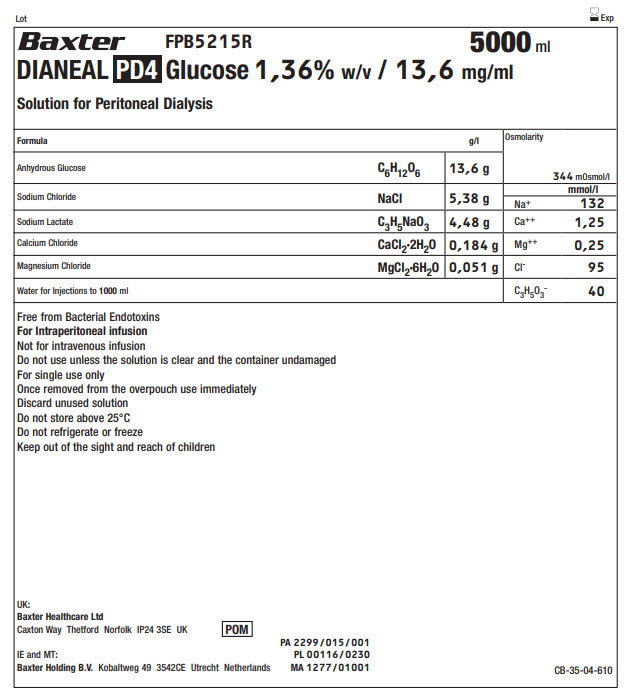
BaxterLogo FPB5215R 5000 ml
DIANEAL PD4 Glucose 1,36% w/v / 13,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
344 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
13,6 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenUK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UKPOM symbol
IE and MT:
Baxter Holding B.V.Kobaltweg 49 354CE Utrecht NetherlandsPA 2299/015/001
PL 00116/0230
MA 1277/01001CB-35-04-610
FPB5215R 5000 ml x 2
Dianeal PD4 Glucose 1.36% w/v / 13,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
13,6 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, NetherlandsPA 2299/015/001 PL 00116/0230 MA 1277/01001
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-198
POM symbol
Barcode
(01)50085412574375(17)201200(10)88B88B88B8Dianeal PD4 Glucose
1.36% w/v / 13,6mg/mlLot:
FPB5215R
5000 ml x 2Lot Exp
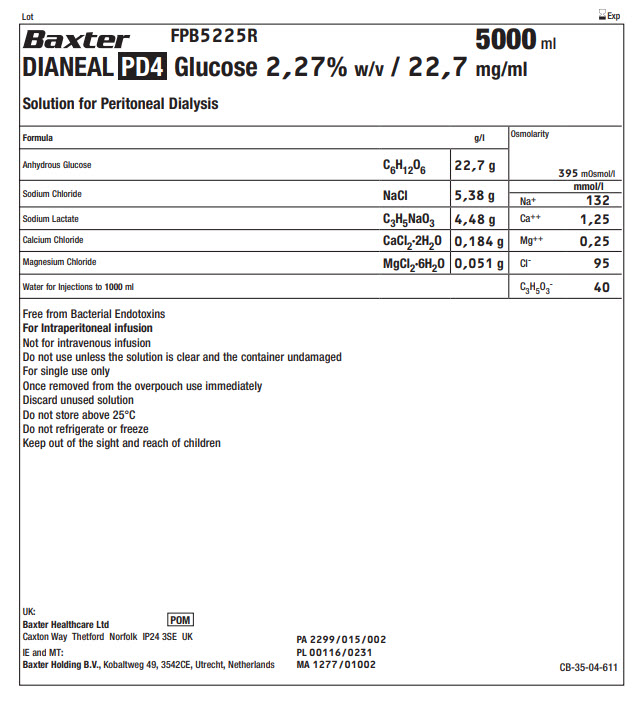
BaxterLogo FPB5225R 5000 ml
DIANEAL PD4 Glucose 2,27% w/v / 22,7 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
395 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
22,7 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenUK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UKPOM symbol
IE and MT:
Baxter Holding B.V.,Kobaltweg 49, 354CE, Utrecht, NetherlandsPA 2299/015/002
PL 00116/0231
MA 1277/01002CB-35-04-611
FPB5225R 5000 ml x 2
Dianeal PD4 Glucose 2.27% w/v / 22,7 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
22,7 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, NetherlandsPA 2299/015/002 PL 00116/0231 MA 1277/01002
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-199
POM symbol
Barcode
(01)50085412574382(17)201200(10)88B88B88B8Dianeal PD4 Glucose
2.27% w/v / 22,7mg/mlLot:
FPB5225R
5000 ml x 2Lot Exp
BaxterLogo FPB5235R 5000 ml
DIANEAL PD4 Glucose 3,86% w/v / 38,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
395 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
38,6 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenUK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UKPOM symbol
IE and MT:
Baxter Holding B.V.Kobaltweg 49 354CE Utrecht NetherlandsPA 2299/015/003
PL 00116/0232
MA 1277/01003CB-35-04-612
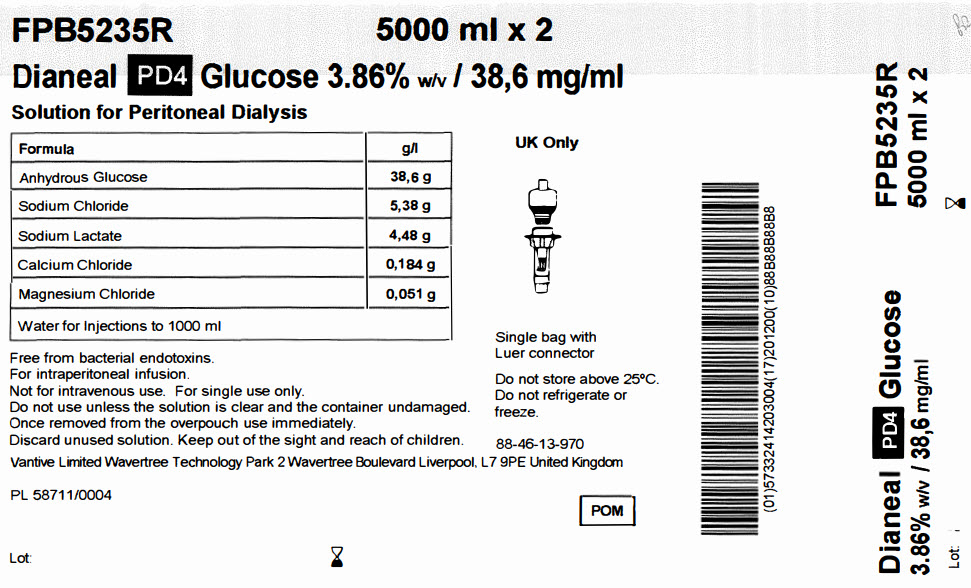
FPB5235R 5000 ml x 2
Dianeal PD4 Glucose 3.86% w/v / 38,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
38,6 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, NetherlandsPA 2299/015/003 PL 00116/0232 MA 1277/01003
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-200
POM symbol
Barcode
(01)50085412574399(17)201200(10)88B88B88B8Dianeal PD4 Glucose
3.86% w/v / 38,6mg/mlLot:
FPB5235R
5000 ml x 2Lot Exp
Vantive Logo FPB5215R 5000 ml
DIANEAL PD4 Glucose 1,36% w/v / 13,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
344 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
13,6 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenVantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United KingdomPOM symbol
PL 58711/0002
CB-35-05-235FPB5215R 5000 ml x 2
Dianeal PD4 Glucose 1.36% w/v / 13,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
13,6 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58711/0002
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-968
POM symbol
Barcode
(01)57332414202991(17)201200(10)88B88B88B8Dianeal PD4 Glucose
1.36% w/v / 13,6mg/mlLot:
FPB5215R
5000 ml x 2Lot Exp
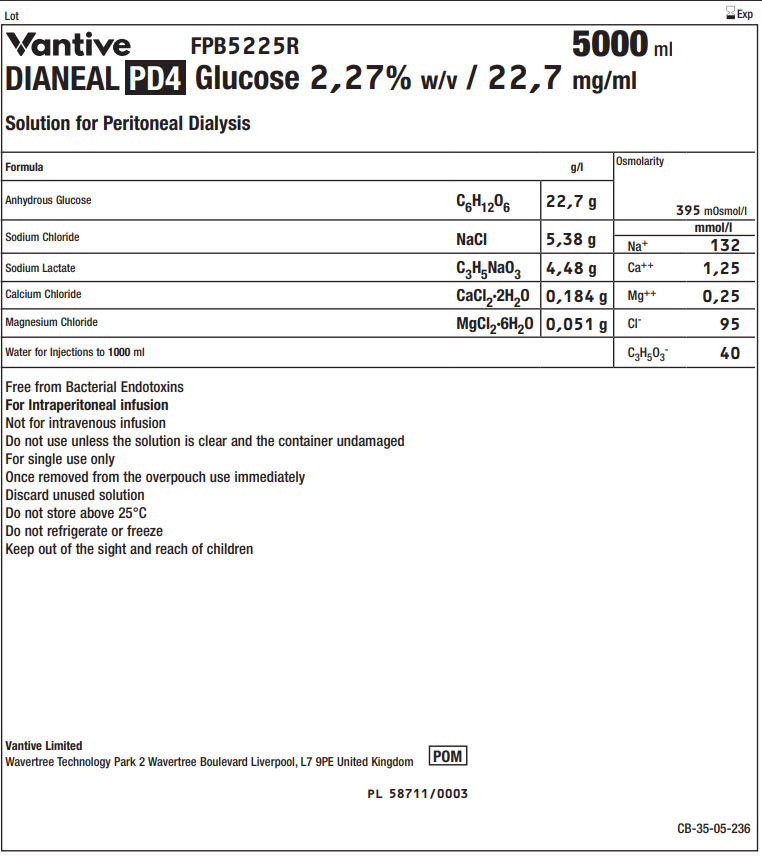
Vantive Logo FPB5225R 5000 ml
DIANEAL PD4 Glucose 2,27% w/v / 22,7 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
395 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
22,7 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenVantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United KingdomPOM symbol
PL 58711/0003
CB-35-05-236
FPB5225R 5000 ml x 2
Dianeal PD4 Glucose 2.27% w/v / 22,7 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
22,7 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58744/0003
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-969
POM symbol
Barcode
(01)57332414203073(17)201200(10)88B88B88B8Dianeal PD4 Glucose
2.27% w/v / 22,7mg/mlLot:
FPB5225R
5000 ml x 2Lot Exp
Vantive Logo FPB5235R 5000 ml
DIANEAL PD4 Glucose 3,86% w/v / 38,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Osmolarity
395 mOsmol/l
Anhydrous Glucose
C 6H 12O 6
38,6 g
Sodium Chloride
NaCl
5,38 g
Na +
mmol/l
132
Sodium Lactate
C 3H 5NaO 3
4,48 g
Ca ++
1,25
Calcium Chloride
CaCl 2·2H 2O
0,184 g
Mg ++
0,25
Magnesium Chloride
MgCl 2·6H 2O
0,051 g
Cl -
95
Water for Injections to 1000ml
C 3H 5O 3-
40
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of childrenVantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United KingdomPOM symbol
PL 58711/0004
CB-35-05-237
FPB5235R 5000 ml x 2
Dianeal PD4 Glucose 3.86% w/v / 38,6 mg/ml
Solution for Peritoneal DialysisFormula
g/l
Anhydrous Glucose
38,6 g
Sodium Chloride
5,38 g
Sodium Lactate
4,48 g
Calcium Chloride
0,184 g
Magnesium Chloride
0,051 g
Water for Injections to 1000 ml
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58711/0004
Lot:
Single bag with
Luer connectorDo not store above 25°C.
Do not refrigerate or
freeze.88-46-13-970
POM symbol
Barcode
(01)57332414203004(17)201200(10)88B88B88B8Dianeal PD4 Glucose
3.86% w/v / 38,6mg/mlLot:
FPB5235R
5000 ml x 2 -
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0727 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0727-02 2 in 1 CARTON 10/29/2024 1 NDC:0941-0727-01 5000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/29/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0729 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0729-02 2 in 1 CARTON 10/29/2024 1 NDC:0941-0729-01 5000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/29/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0731 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0731-02 2 in 1 CARTON 10/29/2024 1 NDC:0941-0731-01 5000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/29/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare S.A. 988899845 analysis(0941-0727, 0941-0729, 0941-0731) , label(0941-0727, 0941-0729, 0941-0731) , manufacture(0941-0727, 0941-0729, 0941-0731) , pack(0941-0727, 0941-0729, 0941-0731) , sterilize(0941-0727, 0941-0729, 0941-0731)