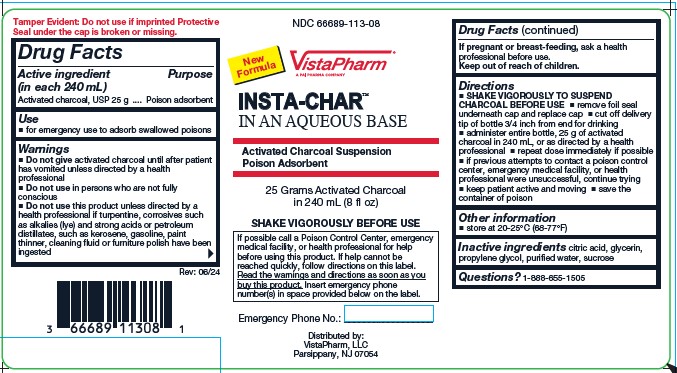
Label: INSTA-CHAR AQUEOUS- poison treatment adsorbent suspension
- NDC Code(s): 66689-113-08
- Packager: VistaPharm, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each 240 mL)
Activated charcoal, USP 25 g
-
Purpose
Poison adsorbent
-
Use
for emergency use to adsorb swallowed poisons
-
Warnings
Do not give activated charcoal until after patient has vomited unless directed by a health professional - Do not use in persons who are not fully conscious - Do not use this product unless ...
-
Directions
SHAKE VIGOROUSLY TO SUSPEND CHARCOAL BEFORE USE - remove foil seal underneath cap and replace cap - cut off delivery tip of bottle 3/4 inch from end for drinking - administer entire bottle, 25 g of ...
-
Other informationstore at 20-25ºC (68-77ºF)
-
Inactive Ingredients
citric acid, glycerin, propylene glycol, purified water, sucrose
-
Questions?
1-888-655-1505
-
PRINCIPAL DISPLAY PANEL-LABELNDC 66689-113-08 - INSTA-CHAR™ IN AN AQUEOUS BASE - Activated Charcoal Suspension - Poison Adsorbent - Distributed by: VistaPharm Inc. Parsippany, NJ 07054
-
INGREDIENTS AND APPEARANCEProduct Information