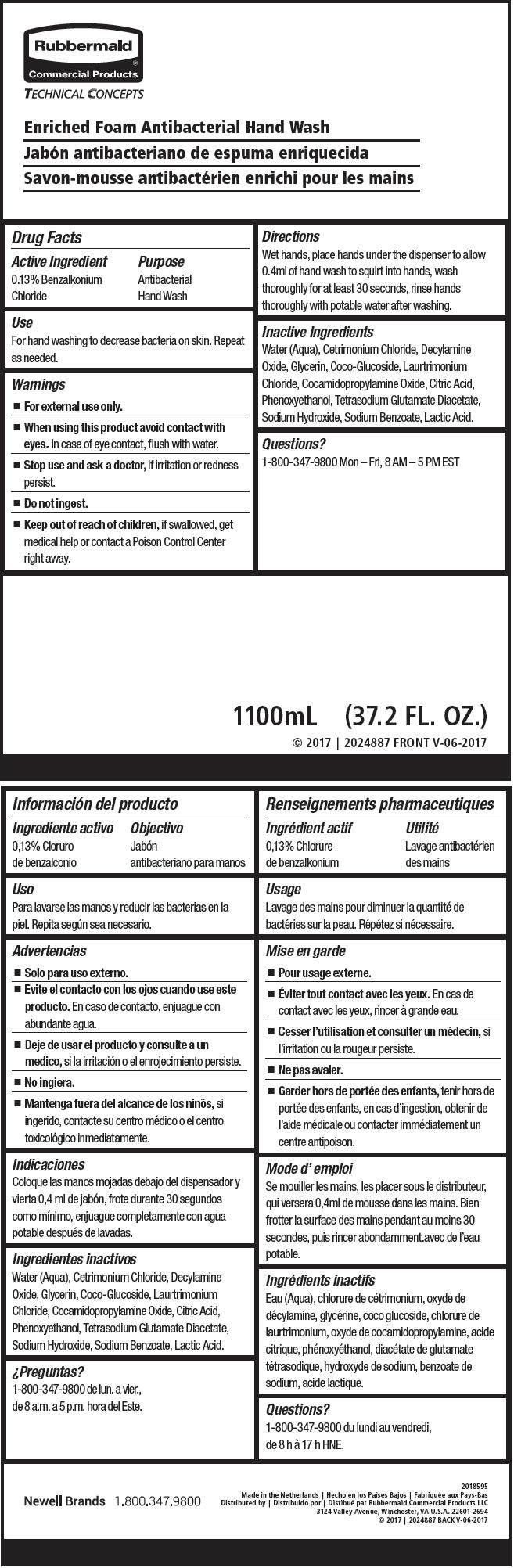
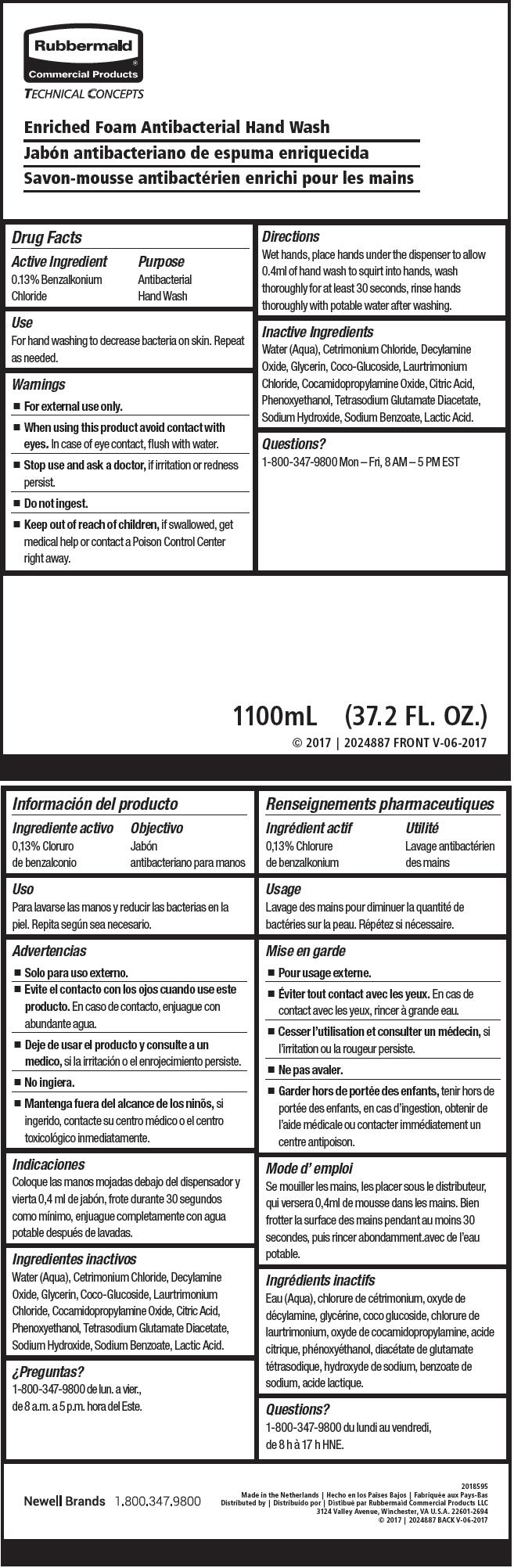
Label: ANTIBACTERIAL HAND WASH- benzalkonium chloride soap
-
NDC Code(s):
65321-032-01,
65321-032-02,
65321-032-03,
65321-032-04, view more65321-032-05
- Packager: Rubbermaid Commercial Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1100 mL Bag Label
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL HAND WASH
benzalkonium chloride soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65321-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 13 mg in 10 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) DECYLAMINE OXIDE (UNII: G387VUT5EZ) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) LAURTRIMONIUM CHLORIDE (UNII: A81MSI0FIC) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENOXYETHANOL (UNII: HIE492ZZ3T) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM BENZOATE (UNII: OJ245FE5EU) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65321-032-01 400 mL in 1 BAG; Type 0: Not a Combination Product 07/24/2017 2 NDC:65321-032-02 800 mL in 1 BAG; Type 0: Not a Combination Product 07/24/2017 3 NDC:65321-032-03 1100 mL in 1 BAG; Type 0: Not a Combination Product 07/24/2017 4 NDC:65321-032-04 1300 mL in 1 BAG; Type 0: Not a Combination Product 07/24/2017 5 NDC:65321-032-05 500 mL in 1 BAG; Type 0: Not a Combination Product 01/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M003 07/24/2017 Labeler - Rubbermaid Commercial Products LLC (049924368) Establishment Name Address ID/FEI Business Operations NWL Netherlands Services B.V. 494692088 ANALYSIS(65321-032) , MANUFACTURE(65321-032)