Label: G TUSSIN AC- codeine phosphate and guaifenesin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57963-103-04, 57963-103-16 - Packager: The Generic Pharmaceutical Company
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 12, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
When using this product
- giving a higher dose than recommended by a doctor could result in serious side effects for your child. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
- may cause or aggravate constipation
- Directions
- Other information
- Question? Comments?
- Inactive ingredients
-
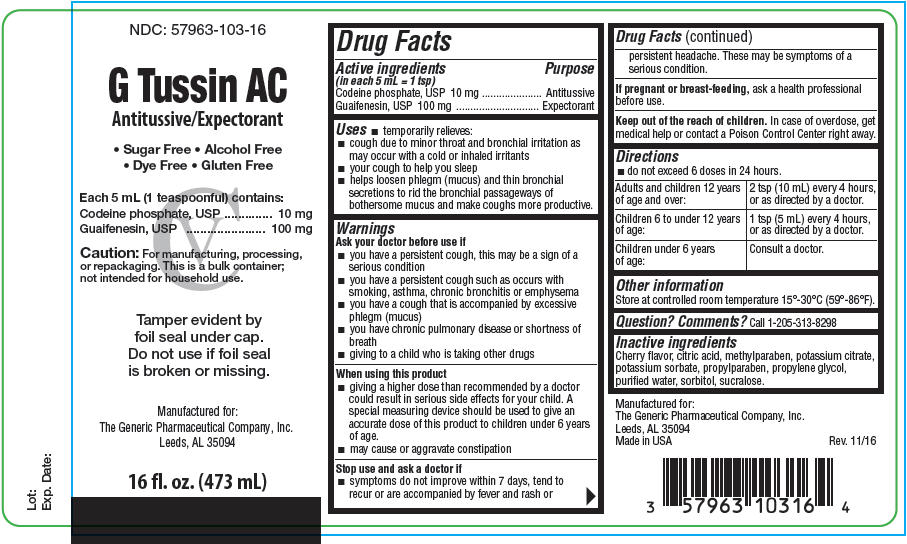
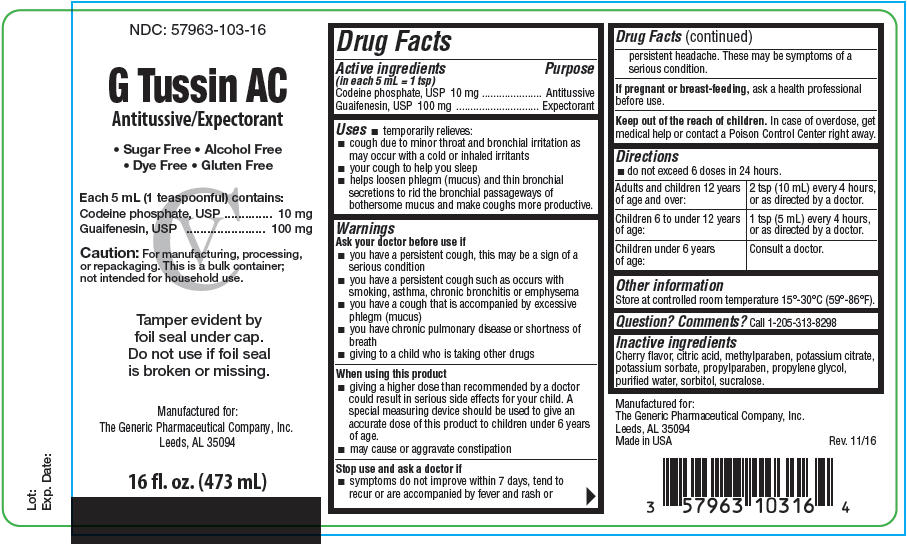
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC: 57963-103-16
G Tussin AC
Antitussive/Expectorant• Sugar Free • Alcohol Free
• Dye Free • Gluten FreeEach 5 mL (1 teaspoonful) contains:
Codeine phosphate, USP 10 mg
Guaifenesin, USP 100 mgCaution: For manufacturing, processing,
or repackaging. This is a bulk container;
not intended for household use.Tamper evident by
foil seal under cap.
Do not use if foil seal
is broken or missing.Manufactured for:
The Generic Pharmaceutical Company, Inc.
Leeds, AL 3509416 fl. oz. (473 mL)
-
INGREDIENTS AND APPEARANCE
G TUSSIN AC
codeine phosphate and guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57963-103 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Methylparaben (UNII: A2I8C7HI9T) Potassium Citrate (UNII: EE90ONI6FF) Potassium Sorbate (UNII: 1VPU26JZZ4) Propylparaben (UNII: Z8IX2SC1OH) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Sucralose (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57963-103-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2016 2 NDC:57963-103-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/01/2016 Labeler - The Generic Pharmaceutical Company (078787060)