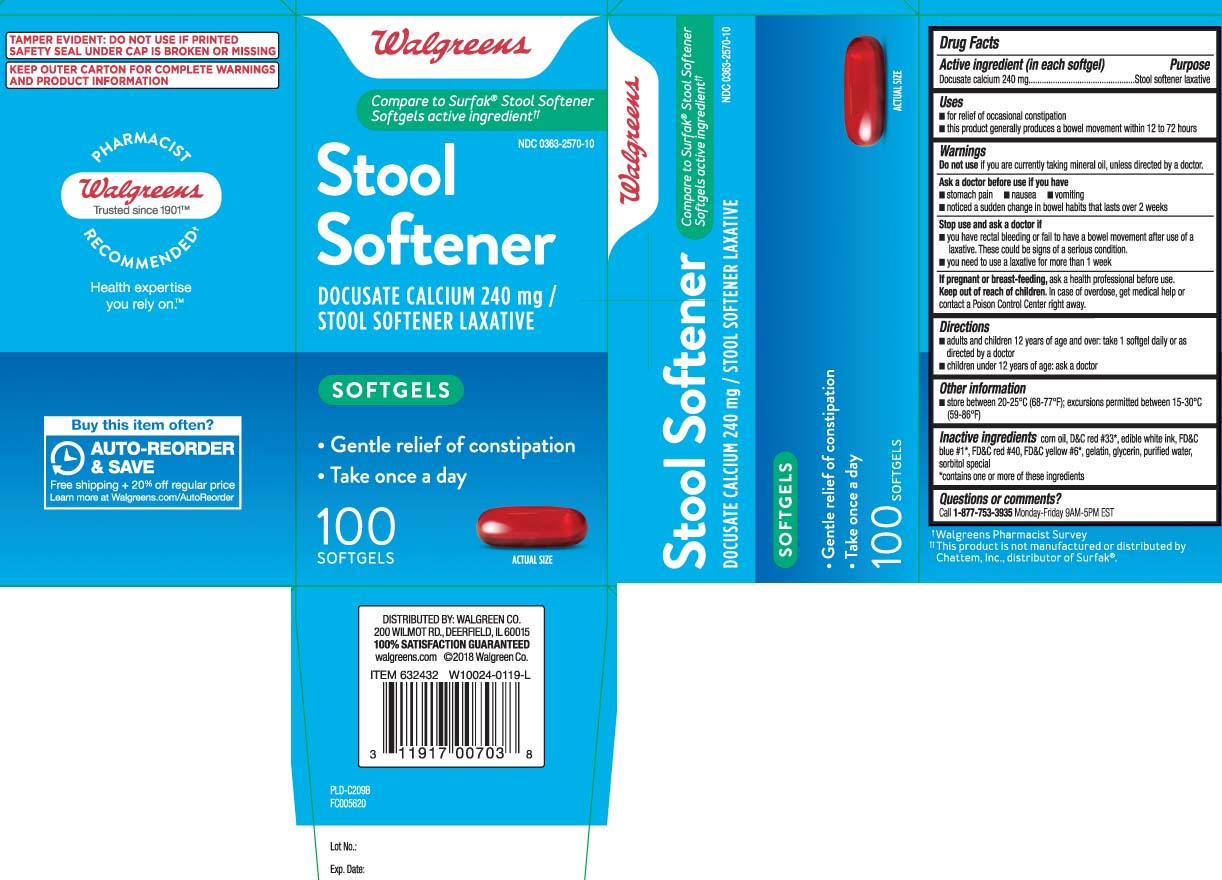
Label: STOOL SOFTENER- docusate calcium capsule, liquid filled
- NDC Code(s): 0363-2570-10
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 29, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each softgel)
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to Surfak® Stool Softener Softgels active ingredient††
Stool Softener
DOCUSATE CALCIUM 240 mg /
STOOL SOFTENER LAXATIVE
SOFTGELS
- Gentle relief of constipation
- Take once a day
SOFTGELS
††This product is not manufactured or distributed by Chattem Inc., distributor of Surfak®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
walgreens.com
- Product Label
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENER
docusate calcium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-2570 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE CALCIUM (UNII: 6K7YS503HC) (DOCUSATE - UNII:M7P27195AG) DOCUSATE CALCIUM 240 mg Inactive Ingredients Ingredient Name Strength CORN OIL (UNII: 8470G57WFM) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SORBITAN (UNII: 6O92ICV9RU) Product Characteristics Color red Score no score Shape CAPSULE Size 18mm Flavor Imprint Code P58;SCU Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-2570-10 1 in 1 BOX 01/31/2019 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 01/31/2019 Labeler - Walgreens (008965063)