Label: NICE N CLEAN WIPES SANI-HANDS ANTIBACTERIAL HAND WIPES- benzalkonium chloride hand wipes swab
-
NDC Code(s):
44385-7014-1,
44385-7014-2,
44385-7014-3,
44385-7014-4, view more44385-7014-5, 44385-7014-6
- Packager: Nice-Pak Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
Flammable, keep away from fire or flame
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or redness develolps and contines for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- REFERENCES
- KEEP OUT OF REACH OF CHILDREN
-
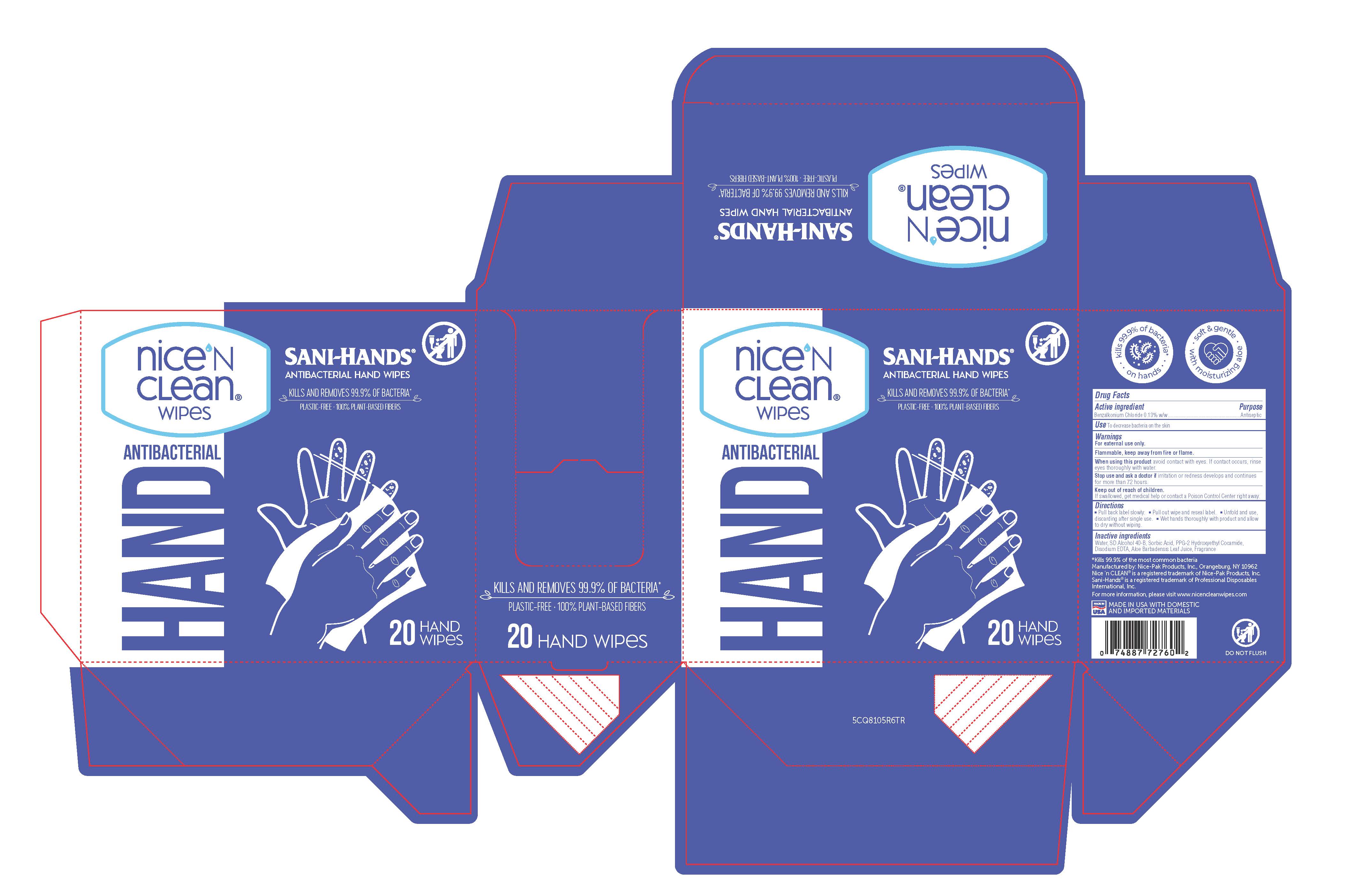
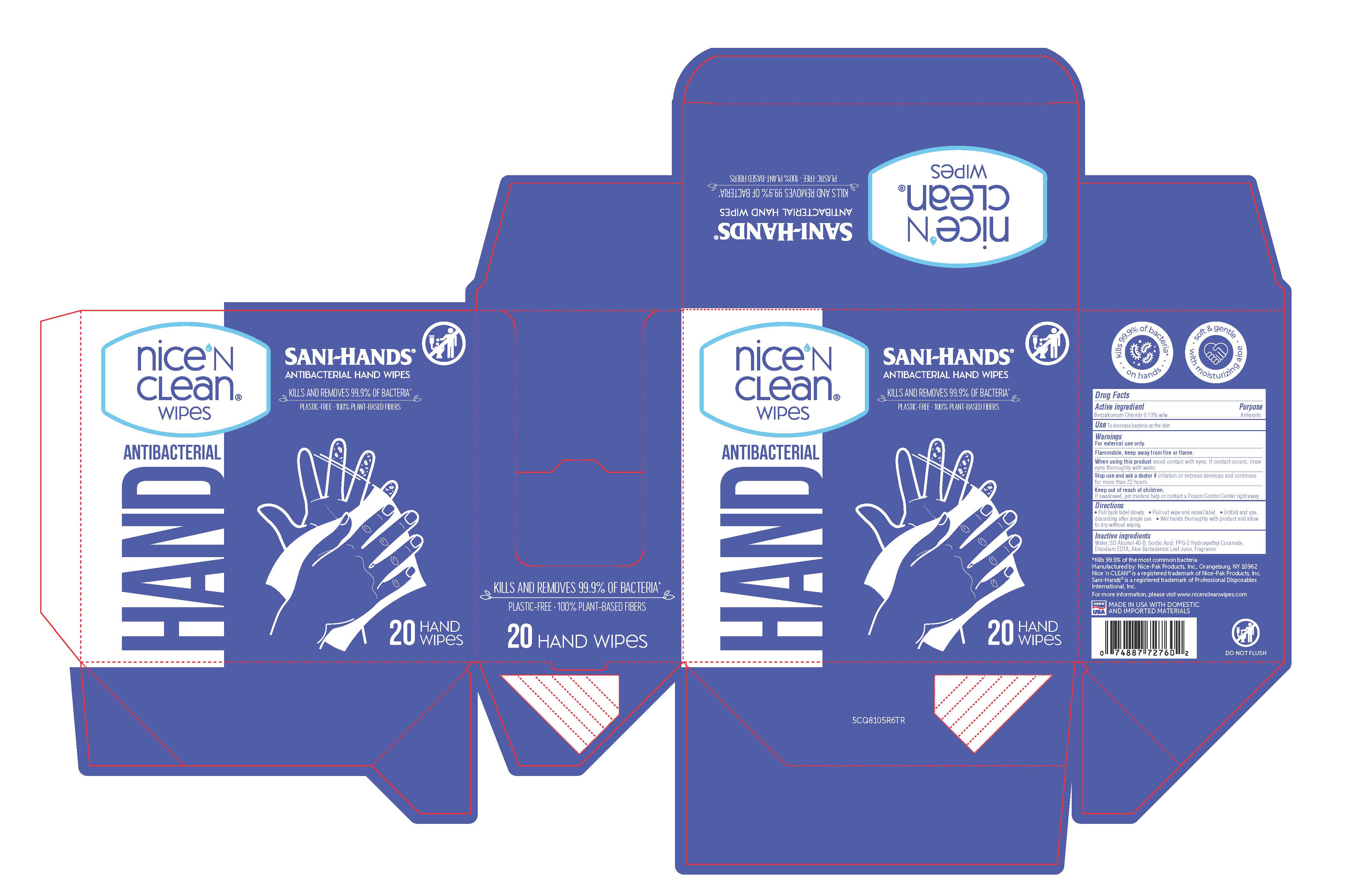
PRINCIPAL DISPLAY PANEL
NICE 'N CLEAN WIPES
SANI-HANDS
ANTIBACTERIAL HAND WIPES
HAND ANTIBACTERIAL
KILLS AND REMOVES 99.9% OF BACTERIA*
DO NOT FLUSH
PLASTIC-FREE - 100% PLANT-BASED FIBERS
*KILLS 99.9% OF THE MOST COMMON BACTERIA
MANUFACTURED BY: NICE-PAK PRODUCTS, INC. ORANGEBURG, NY 10962
NICE 'N clean(R) is a registered trademark of Nice-Pak Products, Inc.
Sani-Hands(R) is a registered trademark of Professional Disposables International, Inc.
MADE IN USA with domestic and imported materials
PLASTIC-FREE MADE WITH PLANT-BASED FIBERS
20 count web:

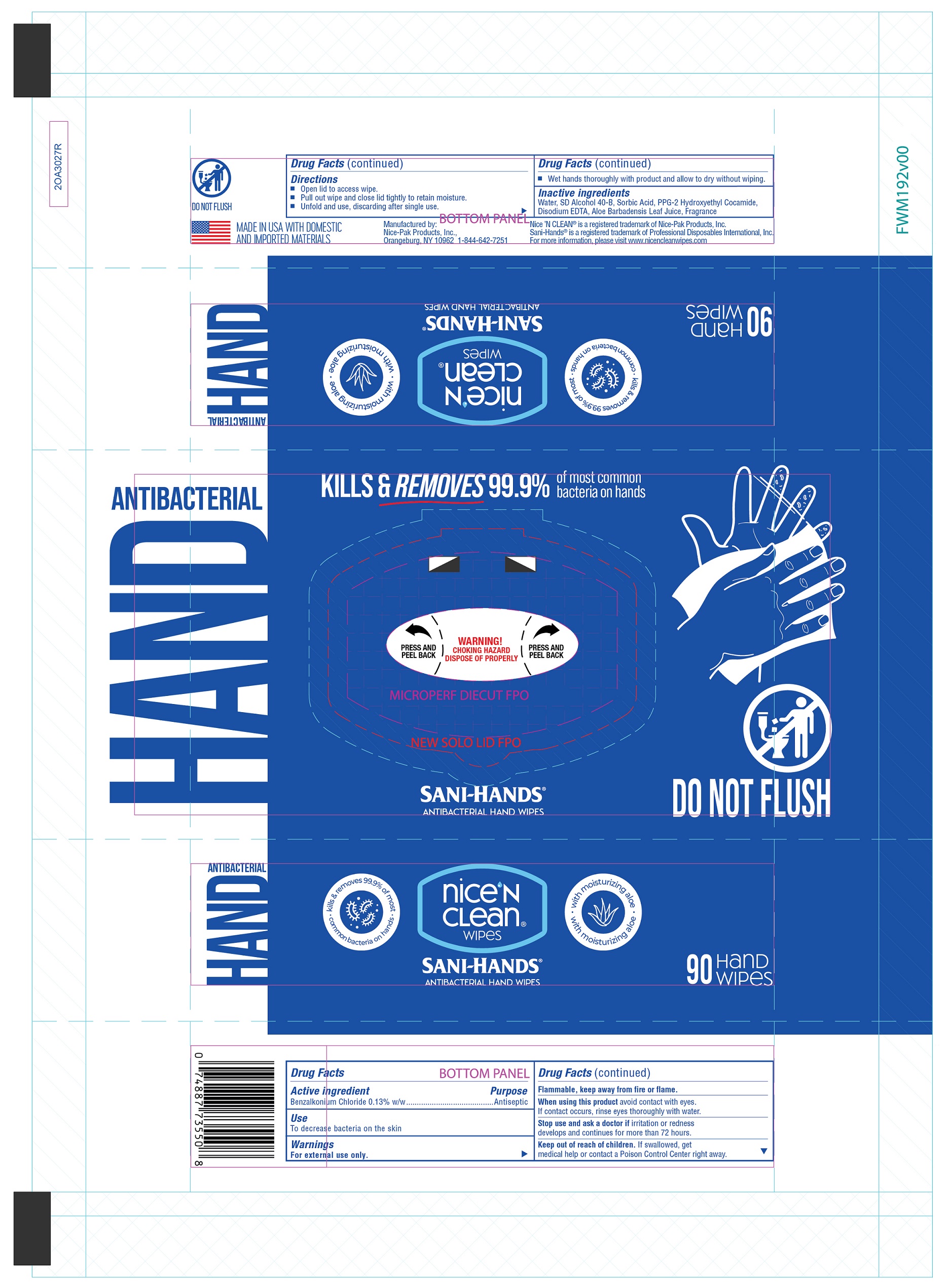
60 count web:

90 Count web:
80 count (4x20ct) in box

320 count (16x20ct) box

-
INGREDIENTS AND APPEARANCE
NICE N CLEAN WIPES SANI-HANDS ANTIBACTERIAL HAND WIPES
benzalkonium chloride hand wipes swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44385-7014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44385-7014-4 261 mL in 1 CELLO PACK; Type 0: Not a Combination Product 12/21/2020 2 NDC:44385-7014-2 175 mL in 1 CELLO PACK; Type 0: Not a Combination Product 12/21/2020 3 NDC:44385-7014-1 53 mL in 1 CELLO PACK; Type 0: Not a Combination Product 12/21/2020 4 NDC:44385-7014-3 4 in 1 BOX 12/21/2020 4 NDC:44385-7014-1 53 mL in 1 CELLO PACK; Type 0: Not a Combination Product 5 NDC:44385-7014-5 6 in 1 TRAY 12/21/2020 5 NDC:44385-7014-1 53 mL in 1 CELLO PACK; Type 0: Not a Combination Product 6 NDC:44385-7014-6 16 in 1 BOX 12/21/2020 6 NDC:44385-7014-1 53 mL in 1 CELLO PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/20/2020 Labeler - Nice-Pak Products, Inc. (003778198) Registrant - Nice-Pak Products, Inc. (003778198) Establishment Name Address ID/FEI Business Operations Nice-Pak Products, Inc. 067900167 manufacture(44385-7014)