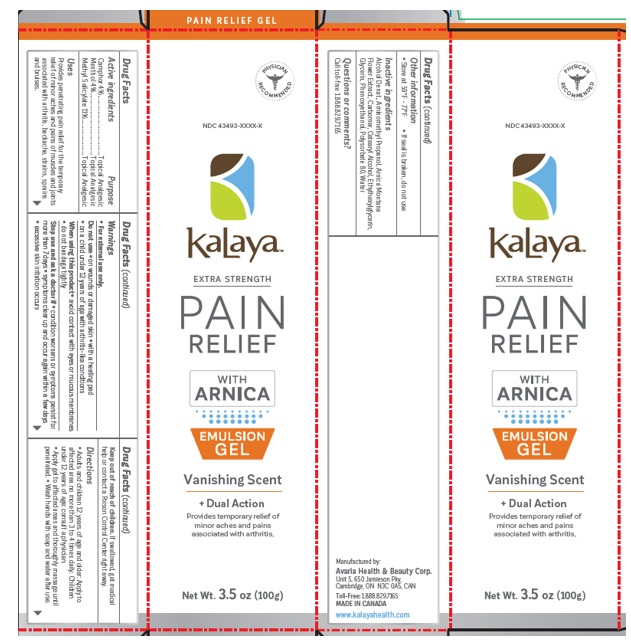
Label: KALAYA EXTRA STRENGTH PAIN RELIEF GEL WITH ARNICA- methyl salicylate, menthol, camphor gel
- NDC Code(s): 43493-0012-0
- Packager: Avaria Health & Beauty Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use- on wounds or damaged skin
- with a heating pad
- on children under 12 years of age with arthritis-like conditions
When using this product
- avoid contact with eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor
- condition worsen or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Cntrol Center right away.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Product label
-
INGREDIENTS AND APPEARANCE
KALAYA EXTRA STRENGTH PAIN RELIEF GEL WITH ARNICA
methyl salicylate, menthol, camphor gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43493-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 11 g in 100 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 4 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43493-0012-0 1 in 1 CARTON 12/01/2023 1 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/16/2023 Labeler - Avaria Health & Beauty Corp (251366043) Establishment Name Address ID/FEI Business Operations Avaria Health & Beauty Corp 251366043 manufacture(43493-0012)