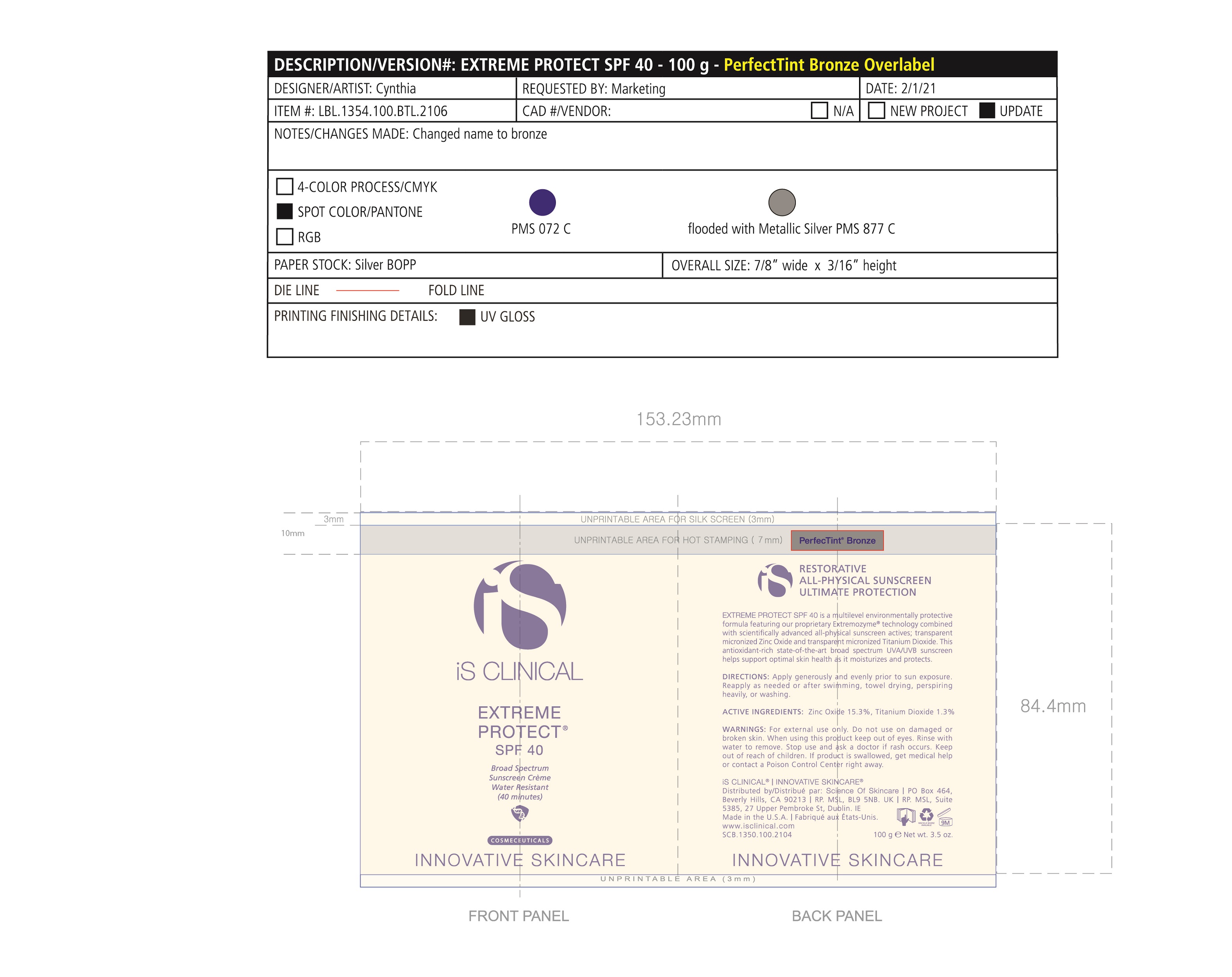
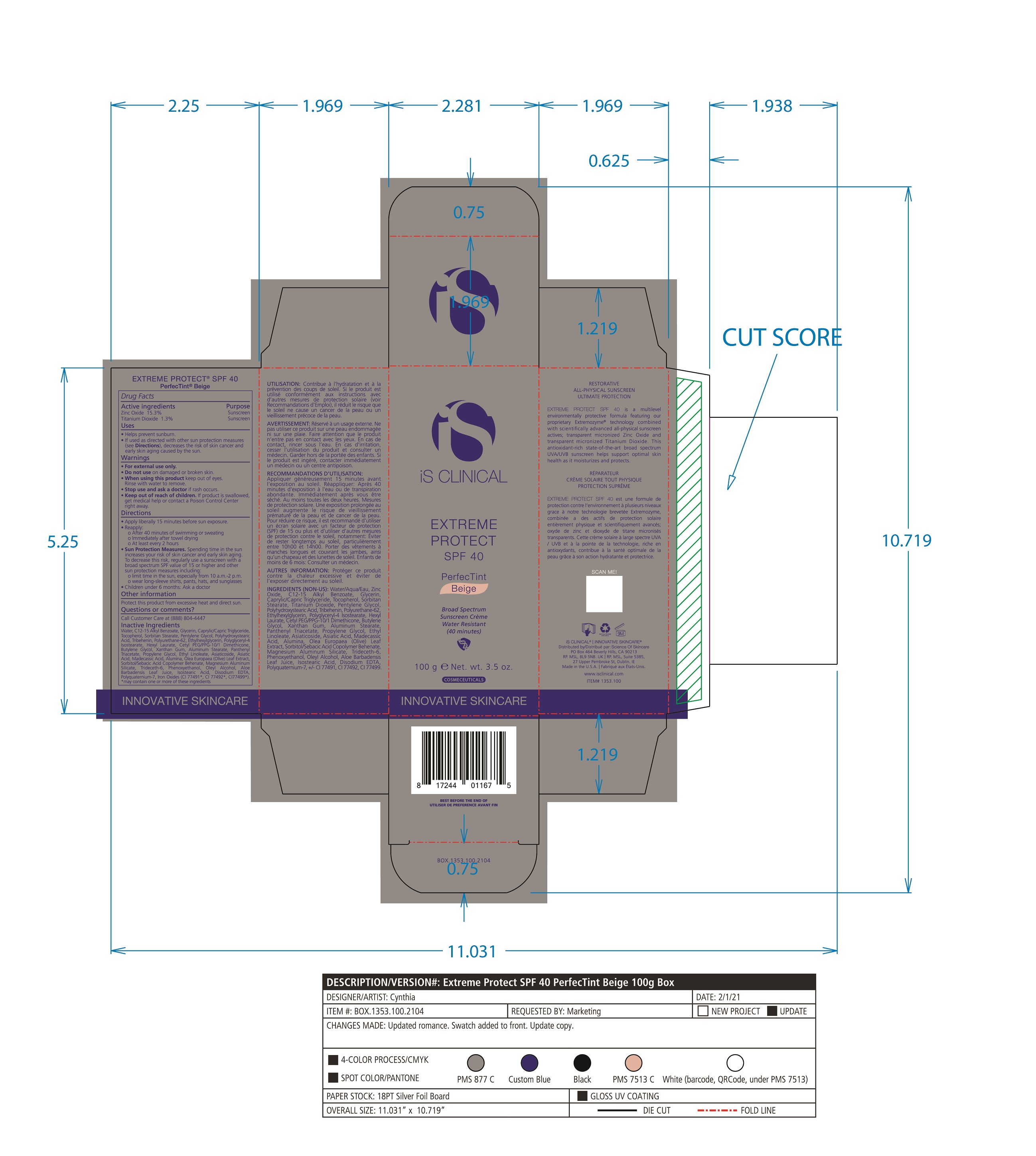
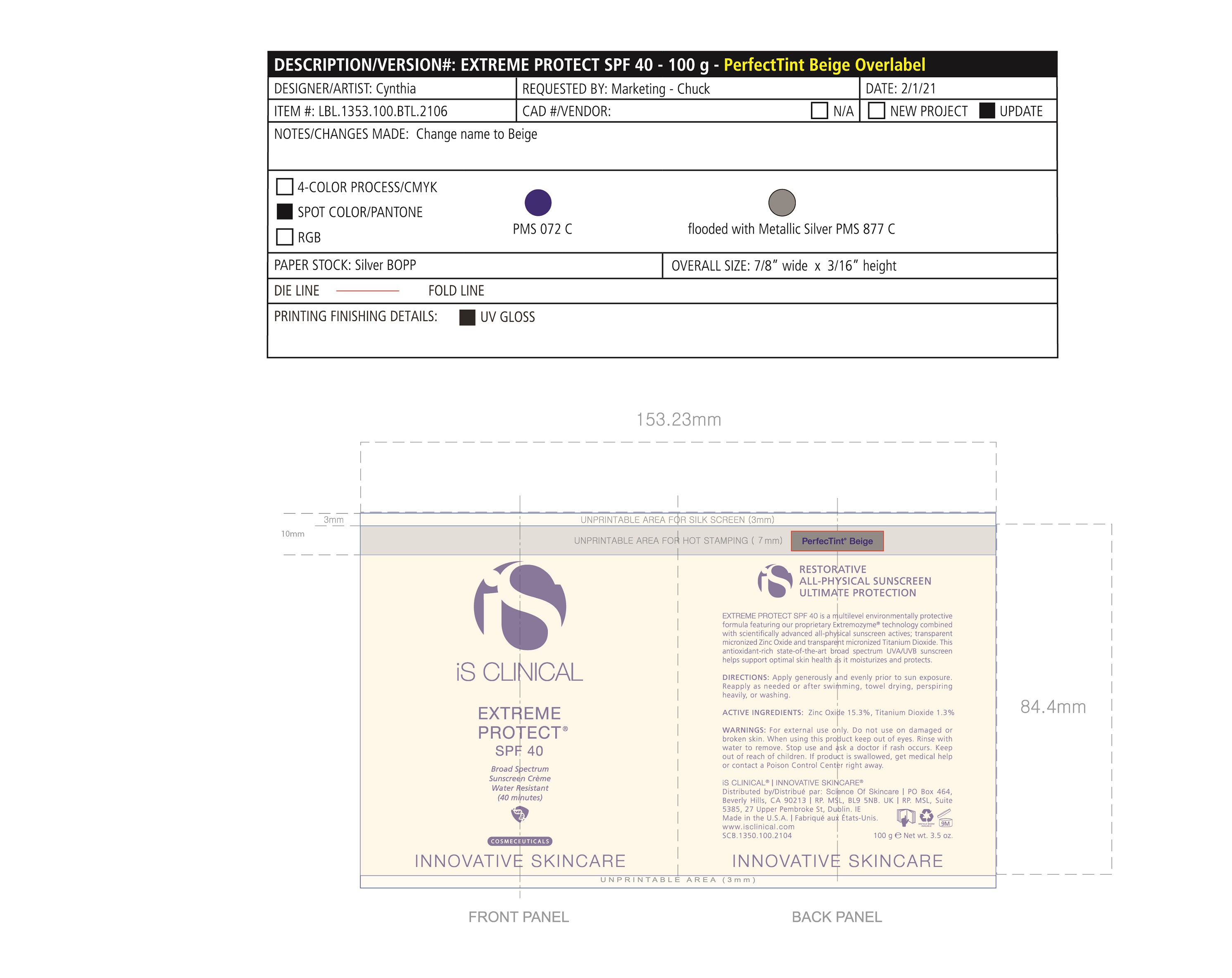
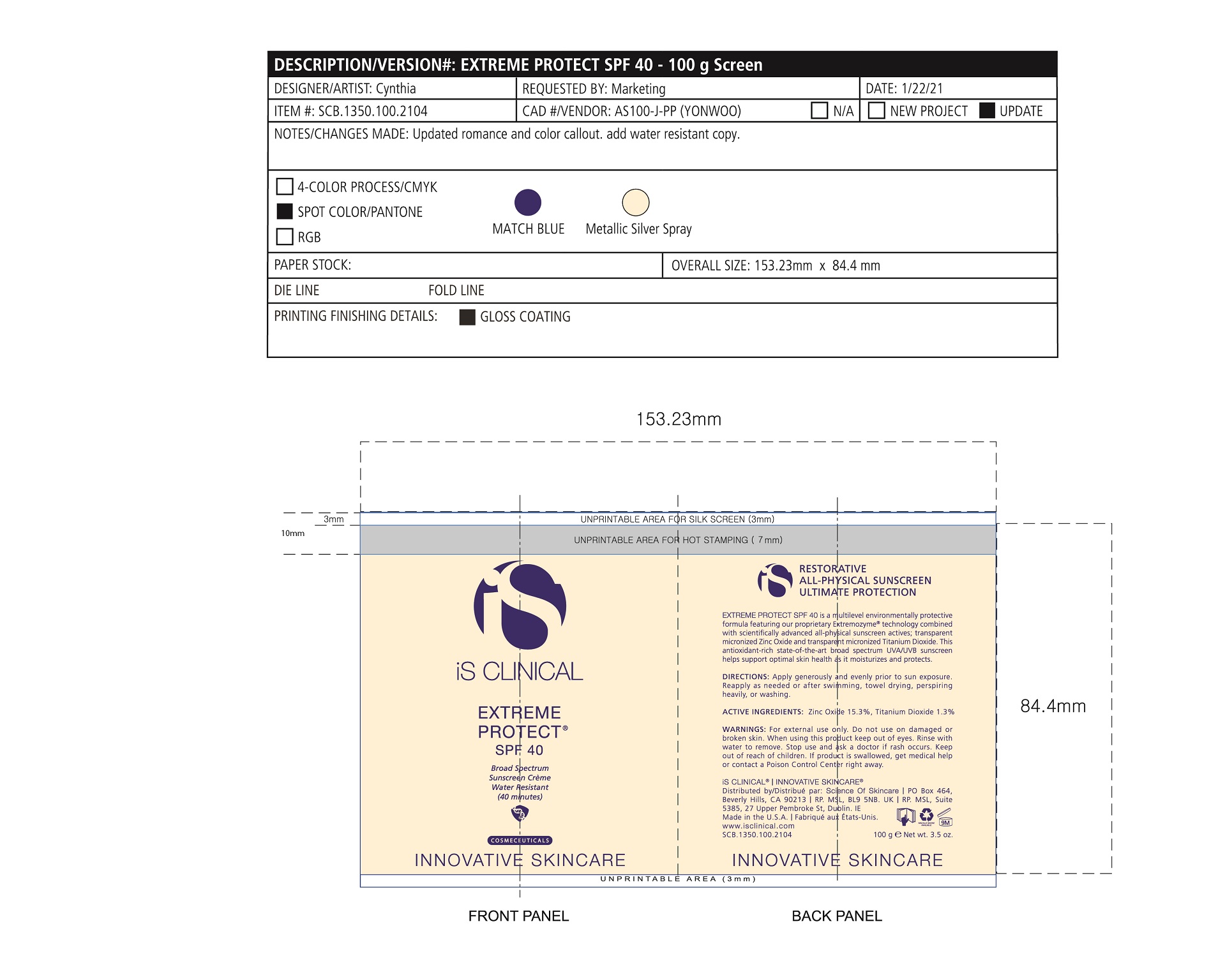
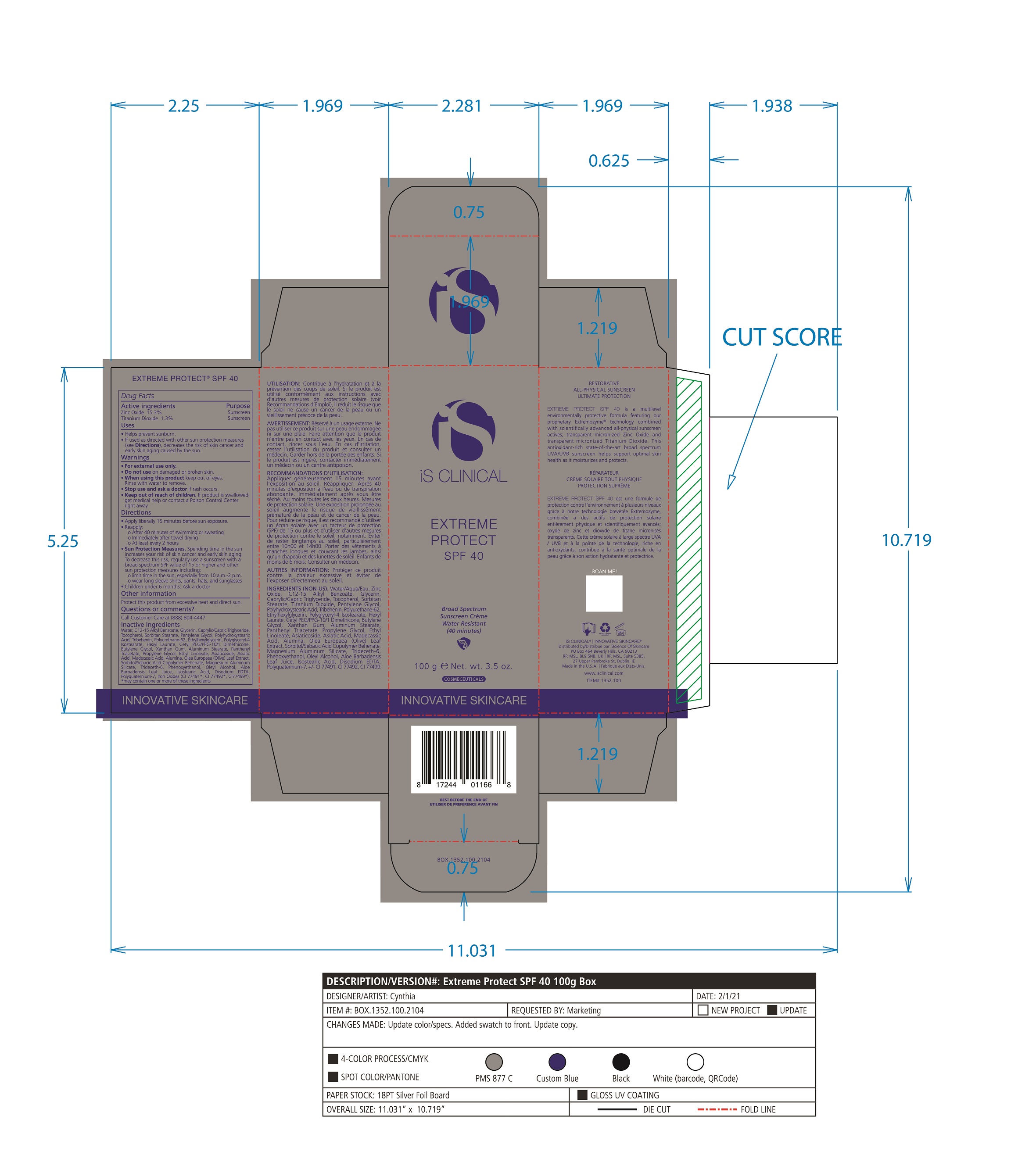
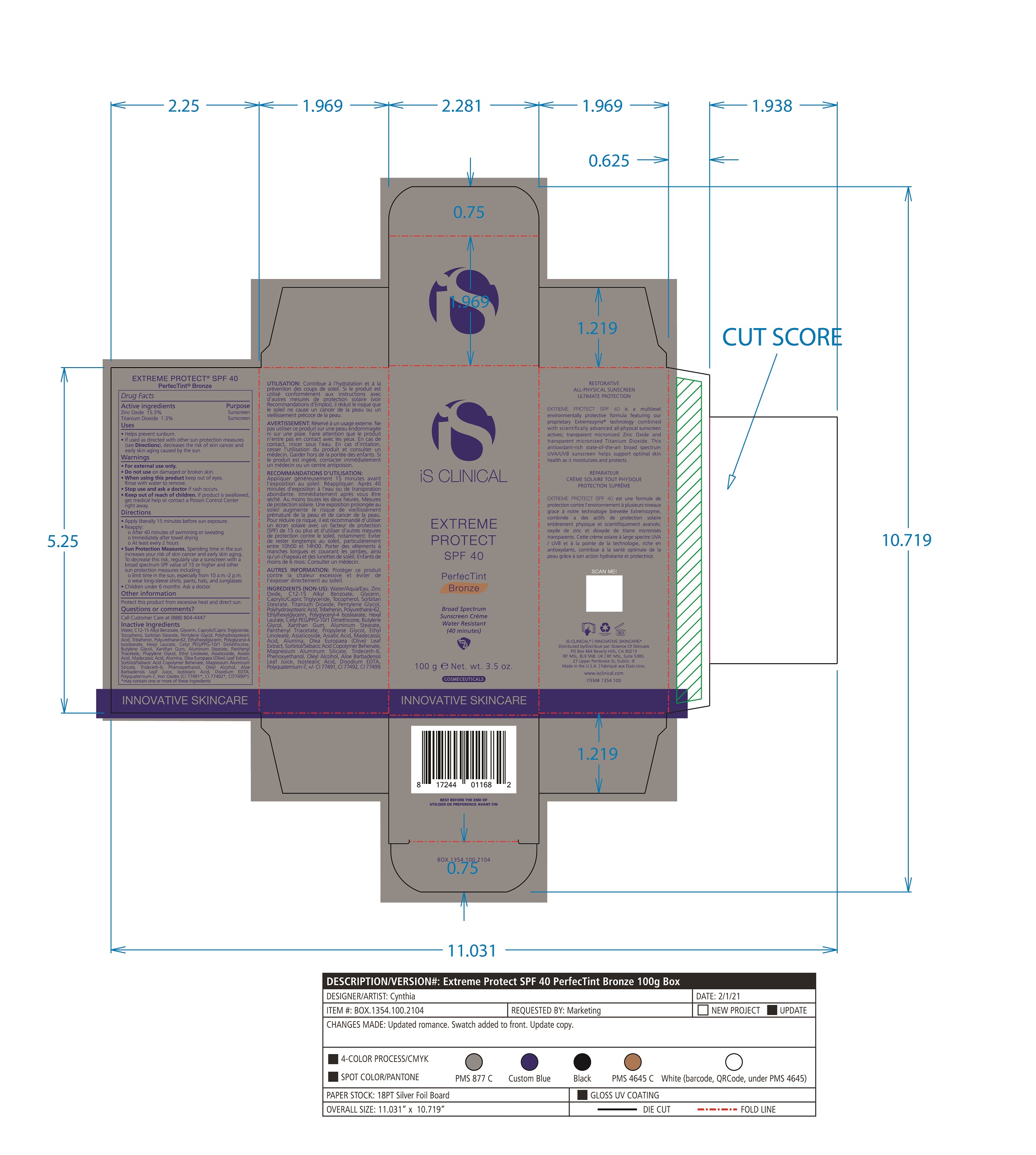
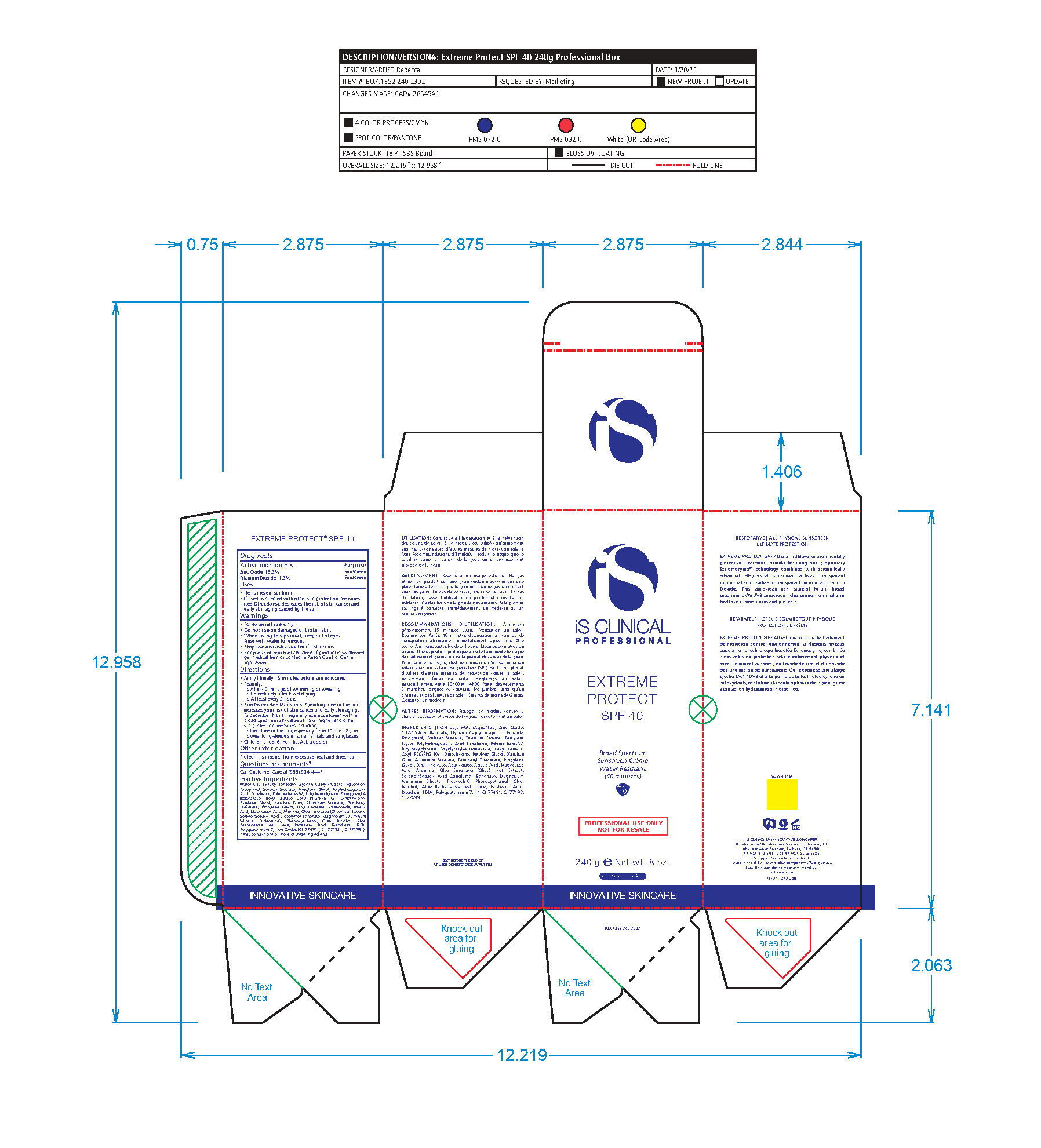
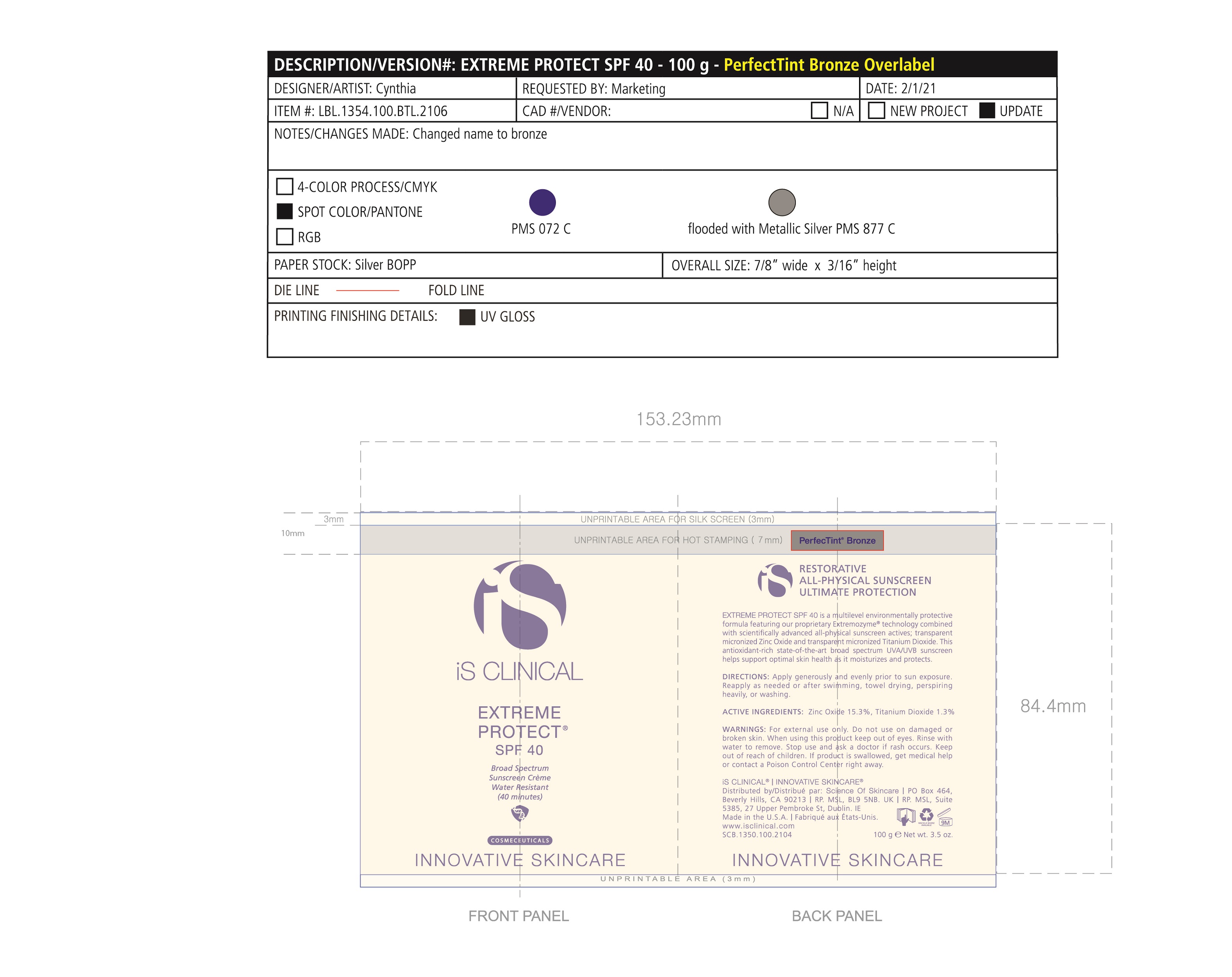
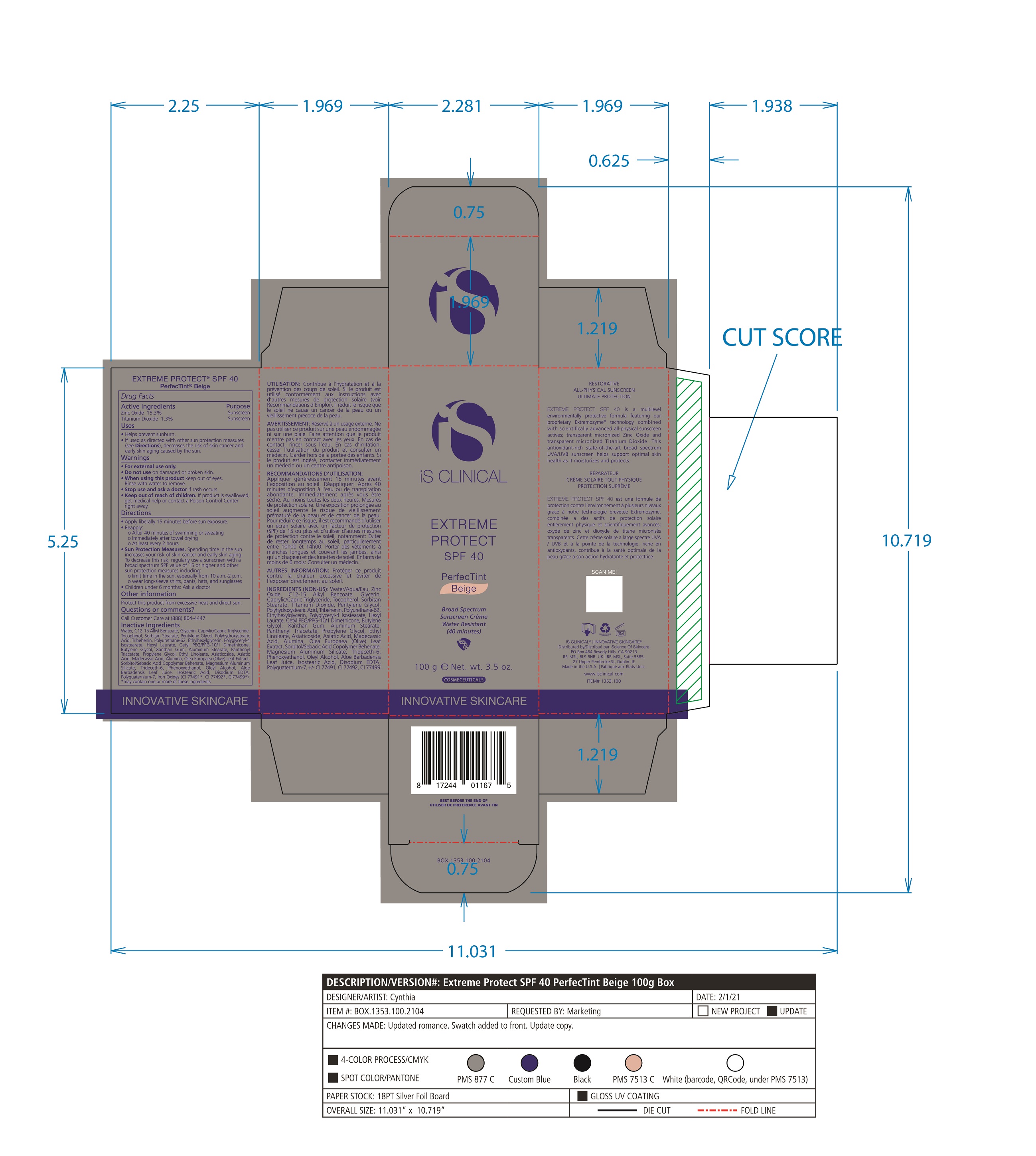
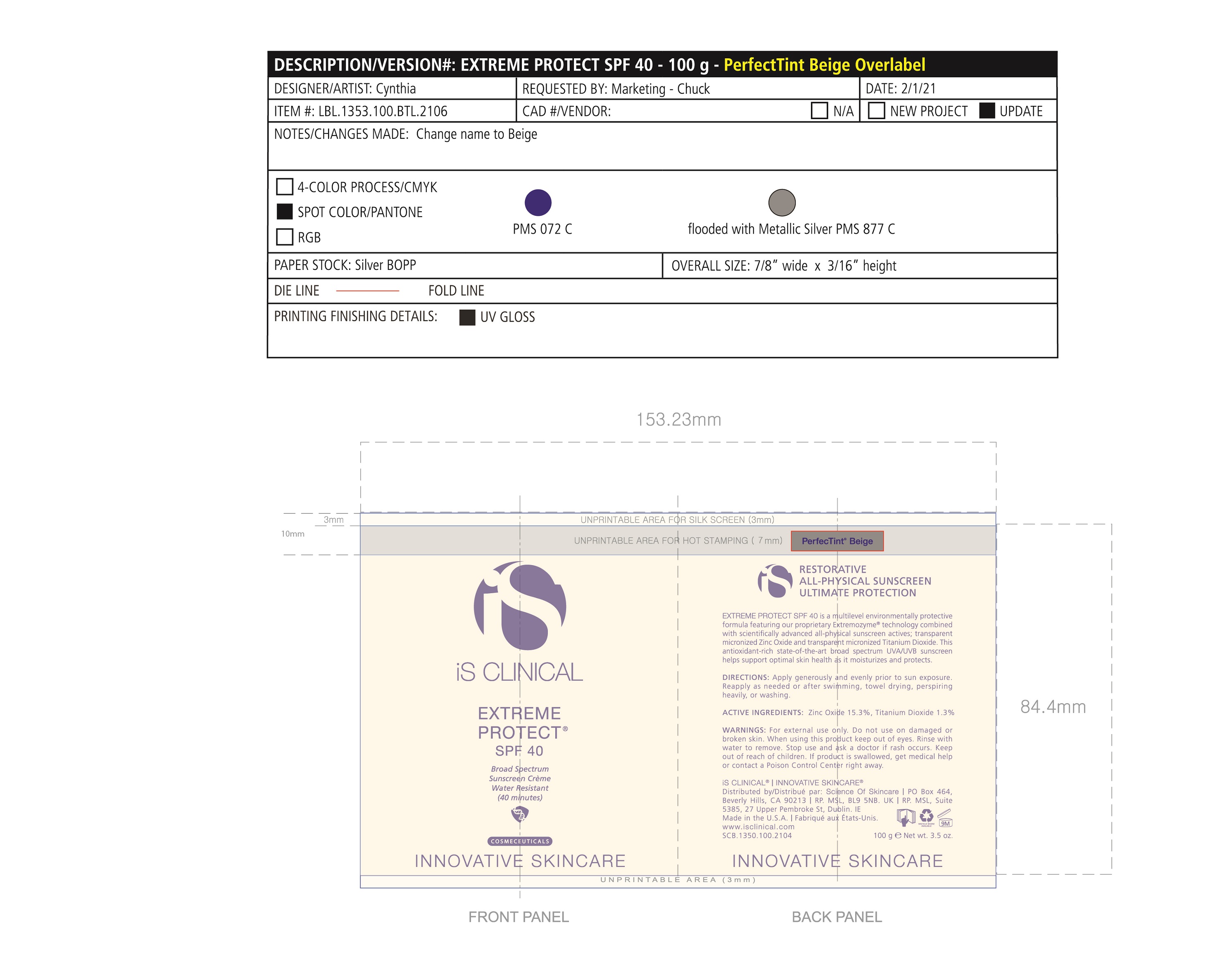
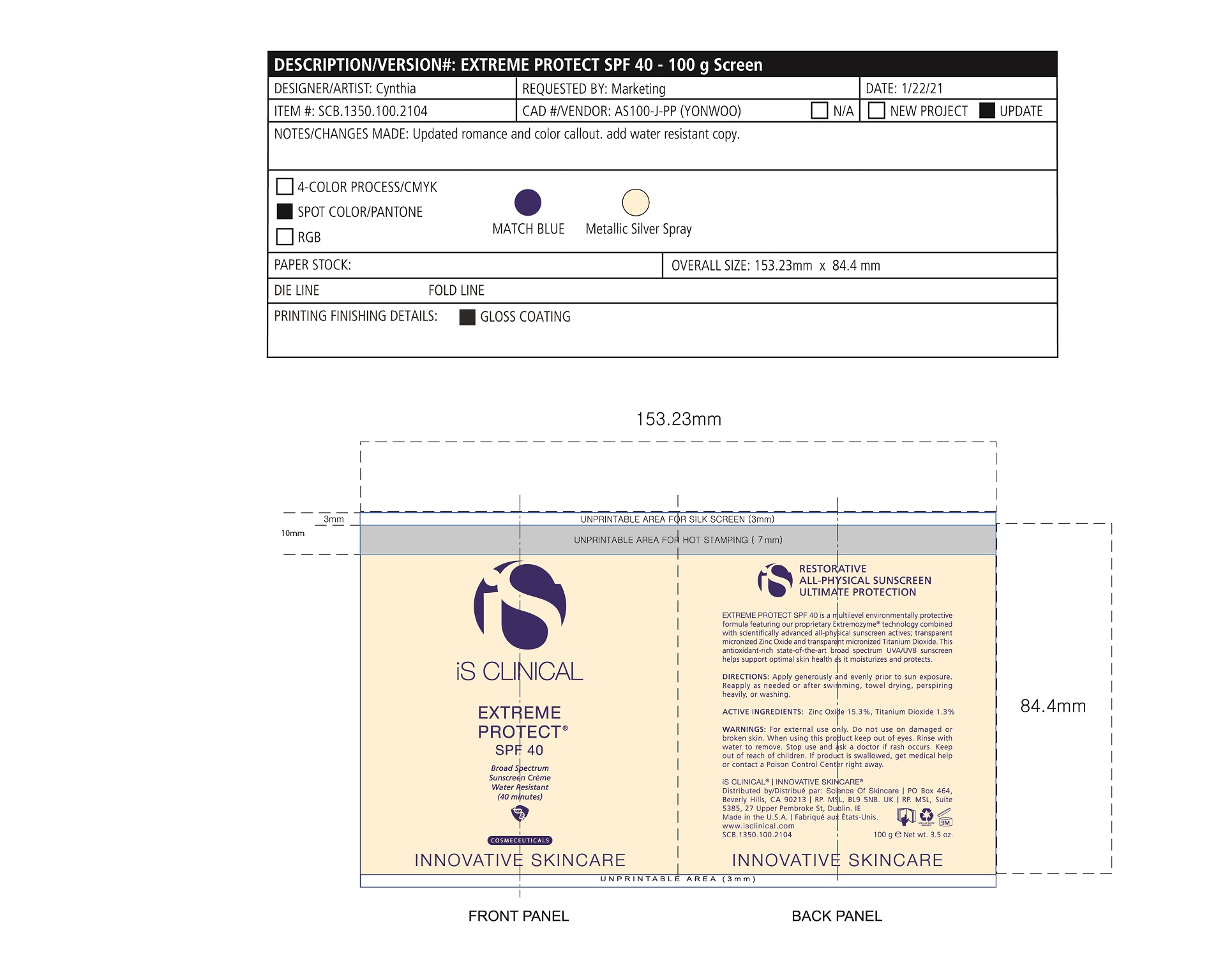
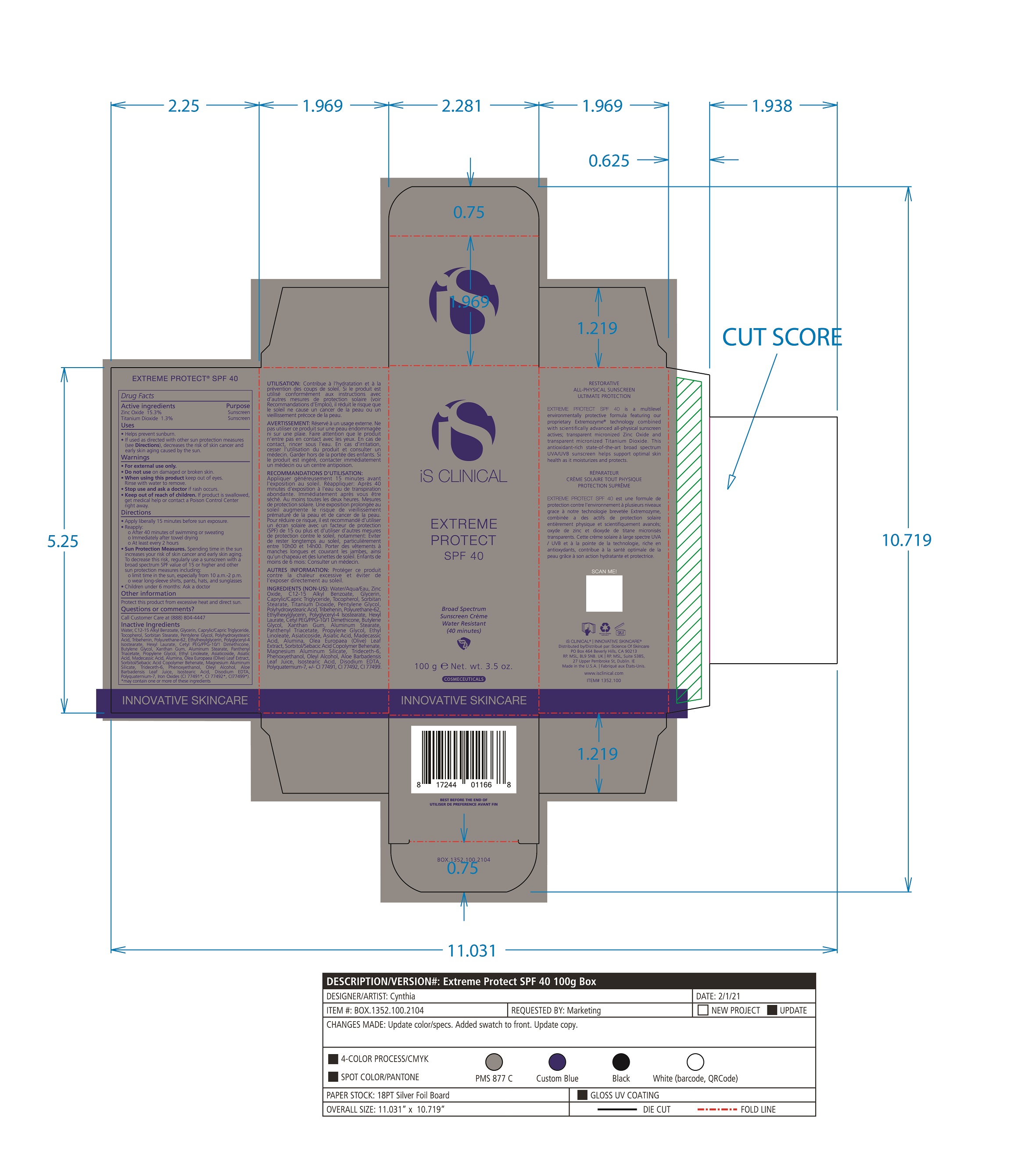
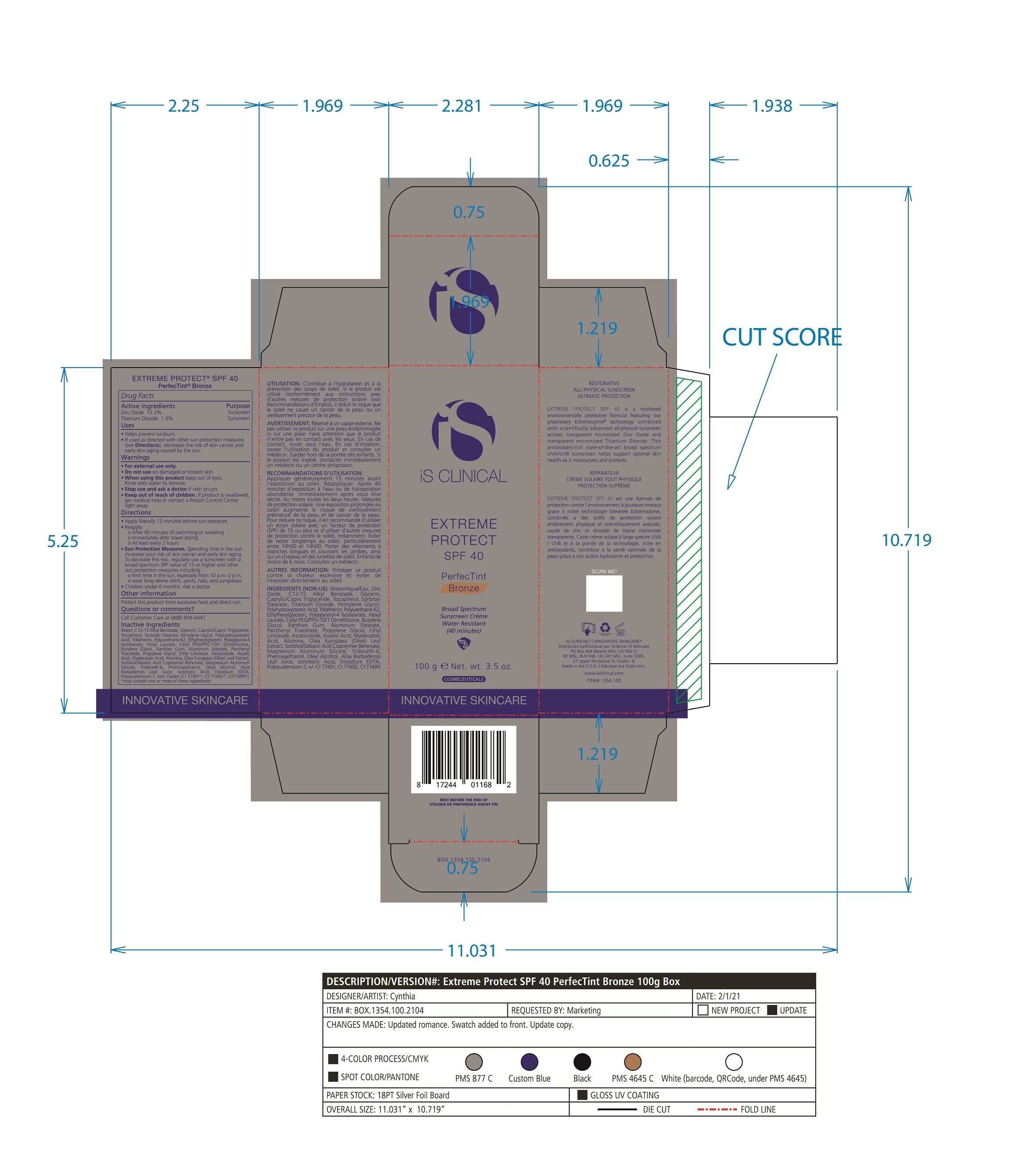
Label: EXTREME PROTECT SPF 40 ALL SHADES- zinc oxide, titanium dioxide cream
-
NDC Code(s):
69219-108-01,
69219-108-02,
69219-108-03,
69219-108-11, view more69219-108-21, 69219-108-31
- Packager: Science of Skincare, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Apply liberally 15 minutes before sun exposure.
Reapply:
After 40 minutes of swimming or sweating
Immediately after towel drying
At least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10am - 2pm.
wear long-sleeve shirts, pants, hats, and sunglasses.
- OTHER SAFETY INFORMATION
- QUESTIONS
-
INACTIVE INGREDIENT
Water/Aqua/Eau, Zinc Oxide, C12-15 Alkyl Benzoate, Glycerin, Caprylic Capric Triglycerides, Tocopherol, Sorbitan Stearate, Panthenyl Triacetate, Titanium Dioxide, Pentylene Glycol, Ethyl Linoleate, Polyhydroxystearic Acid, Tribehenin, Polyurethane-62, Ethylhexylglycerin, Polyglyceryl-4 Isostearate, Hexyl Laurate, Cetyl PEG/PPG-10/1 Dimethicone, Butylene Glycol, Oleyl Alcohol, Xanthan Gum, Aluminum Stearate, Propylene Glycol, Asiaticoside, Asiatic Acid, Madecassic Acid, Alumina, Olea Europaea (Olive) Leaf Extract, Sorbitol/Sebacic Acid Copolymer Behenate, Magnesium Aluminum Silicate, Trideceth-6, Phenoxyethanol, Aloe Barbadensis Leaf Juice, Isostearic Acid, Disodium EDTA, Polyquaternium-7, +/- CI 77491, CI 77492, CI 77499 *May contain one or more of these ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXTREME PROTECT SPF 40 ALL SHADES
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69219-108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.3 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15.3 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYURETHANE-34 (40 MPA, TENSILE STRENGTH OF FILM AT BREAK) (UNII: 77KA3O6NNF) WATER (UNII: 059QF0KO0R) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) ISOSTEARIC ACID (UNII: X33R8U0062) EDETATE DISODIUM (UNII: 7FLD91C86K) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600 KD) (UNII: 0L414VCS5Y) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) OLEYL ALCOHOL (UNII: 172F2WN8DV) MADECASSIC ACID (UNII: M7O1N24J82) DIMETHICONE 100 (UNII: RO266O364U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) ETHYL LINOLEATE (UNII: MJ2YTT4J8M) ASIATICOSIDE (UNII: PKO39VY215) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) HEXYL LAURATE (UNII: 4CG9F9W01Q) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) TRIDECETH-6 (UNII: 3T5PCR2H0C) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ASIATIC ACID (UNII: 9PA5A687X5) TRIBEHENIN (UNII: 8OC9U7TQZ0) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69219-108-11 1 in 1 BOX 04/01/2021 1 NDC:69219-108-01 100 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69219-108-21 1 in 1 BAG 03/02/2021 2 NDC:69219-108-02 5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:69219-108-03 240 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2021 4 NDC:69219-108-01 100 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2021 5 NDC:69219-108-31 1 in 1 BOX 03/01/2021 5 NDC:69219-108-03 240 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2021 Labeler - Science of Skincare, LLC. (006251958)