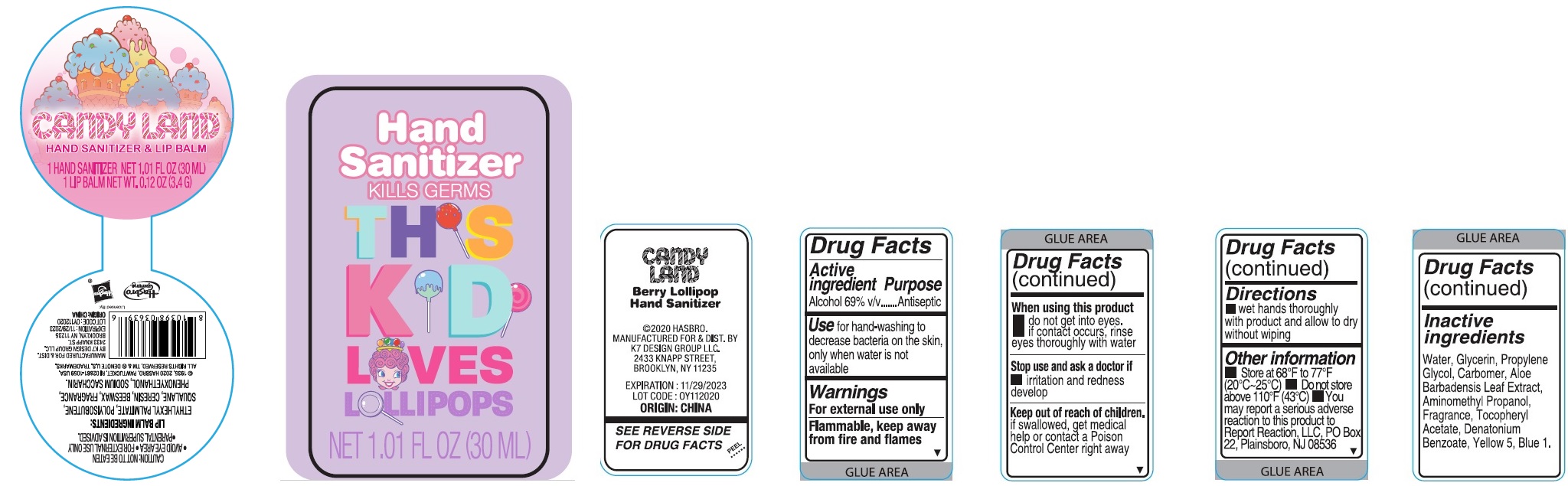
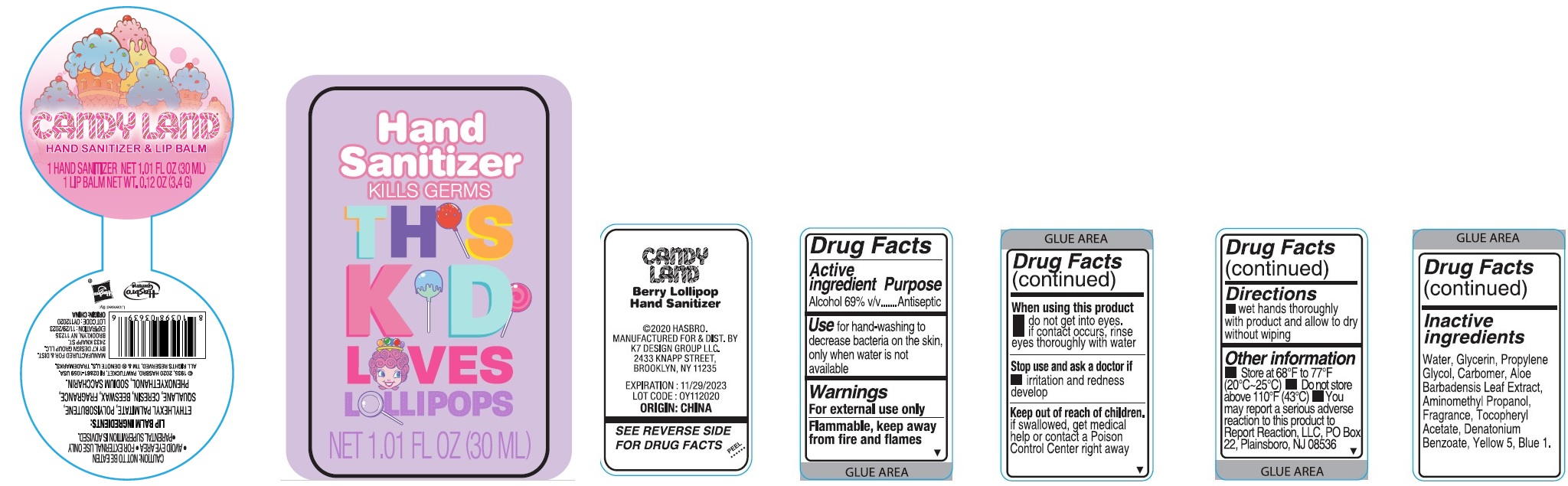
Label: CANDYLAND HAND SANITIZER AND LIP BALM- alcohol kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 74177-967-01 - Packager: K7 Design Group Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Company Information
- Product Packaging
-
INGREDIENTS AND APPEARANCE
CANDYLAND HAND SANITIZER AND LIP BALM
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74177-967 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74177-967-01 1 in 1 KIT; Type 0: Not a Combination Product 12/11/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 30 mL Part 2 1 TUBE 3.4 g Part 1 of 2 BERRY LOLLIPOP HAND SANITIZER
alcohol gelProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 69 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 12/11/2020 Part 2 of 2 LIP BALM
lipstick lipstickProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ETHYLHEXYL PALMITATE (UNII: 2865993309) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR SQUALANE (UNII: GW89575KF9) INGR CERESIN (UNII: Q1LS2UJO3A) INGR YELLOW WAX (UNII: 2ZA36H0S2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 12/11/2020 Labeler - K7 Design Group Inc. (080357784)