Label: SALICYLIC ACID 6 PERCENT- salicylic acid shampoo
- NDC Code(s): 42546-279-06
- Packager: PruGen LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
SHAMPOO contains 6% w/w salicylic acid USP in a vehicle composed of purified water, disodium laureth sulfosuccinate, cocamidopropyl betaine, hexylene glycol, linoleamidpropyl PG-dimonium chloride phosphate, polyquaternium-22, propylene glycol, sodium C14-16 olefin sulfonate, sodium citrate, sodium lauryl sarcosinate, tetrasodium EDTA, tocopherol acetate and fragrance.
Salicylic acid is the 2-hydroxy derivative of benzoic acid having the following structure:
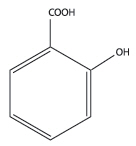
-
CLINICAL PHARMACOLOGY
Salicylic acid has been shown to produce desquamation of the horny layer of skin while not effecting qualitative or quantitative changes in the structure of the viable epidermis. The mechanism of action has been attributed to a dissolution of inter cellular cement substance.
In a study of the percutaneous absorption of salicylic acid in a 6%salicylic acid gel in four patients with extensive active psoriasis, Taylor and Halprin showed that the peak serum salicylate levels never exceeded 5 mg/100 ml even though more than 60% of the applied salicylic acid was absorbed. Systemic toxic reactions are usually associated with much higher serum levels (30 to 40 mg/100 ml).
Peak serum levels occurred within five hours of the topical application under occlusion. The sites were occluded for 10 hours over the entire body surface below the neck. Since salicylates are distributed in the extra cellular space, patients with a contracted extra cellular space due to dehydration or diuretics have a higher salicylate levels than those with a normal extra cellular space. (See PRECAUTIONS)
The major metabolites identified in the urine after topical administration are salicyluric acid (52%), salicylate glucuronides (42%) and free salicylic acid (6%). The urinary metabolites after percutaneous absorption differ from those after oral salicylate administration; those derived from percutaneous absorption contain more salicylate glucuronides and less salicyluric and salicylic acid. Almost 95% of a single dose of salicylate is excreted within 24 hours of its entrance into the extracellular space.
Fifty to eighty percent of a salicylate is protein bound to albumin. Salicylates compete with the binding of several drugs and can modify the actions of these drugs; by similar competitive mechanisms other drugs can influence the serum levels of salicylate.(See PRECAUTIONS)
-
INDICATIONS AND USAGE
For Dermatologic Use
Salicylic Acid 6% (w/w) Shampoo is a topical aid in the removal of excessive keratin in hyperkeratotic skin disorders, including verrucae, and the various ichthyoses (vulgaris, sex-linked and lamellar), keratosis palmaris and plantaris, keratosis pilaris, pityriasis rubra pilaris, and psoriasis (including body, scalp, palms and soles).
- CONTRAINDICATIONS
-
WARNINGS
Prolonged use over large areas, especially in children and those patients with significant renal or hepatic impairment could resultin salicylism. Excessive application of the product other than is needed to cover the affected area will not result in more therapeutic benefit. Concomitant use of other drugs which may contribute to elevated serum salicylate levels should be avoided where the potential for toxicity is present. In children under 12 years of age and those patients with renal or hepatic impairment, the area to be treated should be limited and the patient monitored closely for signs of salicylate toxicity: nausea, vomiting, dizziness, loss of hearing, tinnitus, lethargy, hyperpnea, diarrhea, and psychic disturbances. In the event of salicylic acid toxicity, the use of the Salicylic Acid 6% (w/w) Shampoo should be discontinued. Fluids should be administered to promote urinary excretion. Treatment with sodium bicarbonate (oral or intravenous) should be instituted as appropriate. Patients should be cautioned against the use of oral aspirin and other salicylate containing medications, such as sports and injury creams, to avoid additional excessive exposure to salicylic acid. Where needed, aspirin should be replaced by an alternative non-steroidal, anti-inflammatory agent that is not salicylate based.
Due to potential risk of developing Reye's syndrome, salicylate products should not be used in children and teenagers with varicella or influenza, unless directed by a physician.
-
PRECAUTIONS
For external use only. Avoid contact with eyes and other mucous membranes.
DRUG INTERACTIONS
The following interactions are from a published review and include reports concerning both oral and topical salicylate administration. The relationship of these interactions to the use of Salicylic Acid 6% (w/w) Shampoo is not known.
I.Due to the competition of salicylate with other drugs for binding to serum albumin the following drug interactions may occur:
DRUG DESCRIPTION OF INTERACTION Sulfonylureas Hypoglycemia potentiated. Methotrexate Decreases tubular reabsorption; clinical toxicity from methotrexate can result. Oral Anticoagulants Increased bleeding. II.Drugs changing salicylate levels by altering renal tubular reabsorption:
DRUG DESCRIPTION OF INTERACTION Corticosteroids Decreases plasma salicylate level; tapering doses of steroids may promote salicylism. Acidifying Agents Increases plasma salicylate level. Alkanizing Agents Decreased plasma salicylate levels. III.Drugs with complicated interactions with salicylates:
DRUG DESCRIPTION OF INTERACTION Heparin Salicylate decreases platelet adhesiveness and interferes with hemostasis in heparin treated patients. Pyrazinamide Inhibits pyrazinamide-induced hyperuricemia. Uricosuric Effect of probenemide, sulfinpyrazone and phenylbutazone inhibited. The following alterations of laboratory tests have been reported during salicylate therapy:
LABORATORY TESTS EFFECT OF SALICYLATES Thyroid Function Decreased PBI; increased T3 uptake. Urinary Sugar False negative with glucose oxidase; false positive with Clinitest with high-dose salicylate therapy (2-5g q.d.). 5- Hydroxyindole acetic acid False negative with fluorometric test. Acetone, ketone bodies False positive FeCl3 in Gerhardt reaction; red color persists with boiling .17-OH corticosteroids False reduced values with >4.8g q.d. salicylate. Vanilmandelic acid False reduced values. Uric acid May increase or decrease depending ondose. Prothrombin Decreased levels; slightly increased prothrombin time. Pregnancy (Category C)
Salicylic acid has been shown to be teratogenic in rats and monkeys. It is difficult to extrapolate from oral doses of acetylsalicylic acid used in these studies to topical administration as the oral dose to monkeys may represent six times the maximal daily human dose of salicylic acid when applied topically over a large body surface. There are no adequate and well-controlled studies in pregnant women. Salicylic Acid 6% (w/w) Shampoo should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from the mother's use of Salicylic Acid 6% (w/w) Shampoo, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If used by nursing mothers, it should not be used on the chest area to avoid the accidental contamination of the child.
- ADVERSE REACTIONS
-
OVERDOSAGE
See WARNINGS.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 177 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID 6 PERCENT
salicylic acid shampooProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42546-279 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 6 mg in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) TROLAMINE (UNII: 9O3K93S3TK) QUATERNIUM-22 (UNII: MXO138JCBP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) CHAMOMILE (UNII: FGL3685T2X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42546-279-06 1 in 1 BOX 01/01/2009 1 177 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2009 Labeler - PruGen LLC (929922750) Establishment Name Address ID/FEI Business Operations EMS ACQUISITION CORP. 048602791 MANUFACTURE(42546-279)


