Label: DEMECLOCYCLINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-0155-1 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 65162-554
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Full Prescribing Information
To reduce the development of drug-resistant bacteria and maintain the effectiveness of demeclocycline hydrochloride tablets and other antibacterial drugs, demeclocycline hydrochloride tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
Demeclocycline hydrochloride is an antibiotic isolated from a mutant strain of . Chemically it is 7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12, 12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride. Its structural formula is: Streptomyces aureofaciens
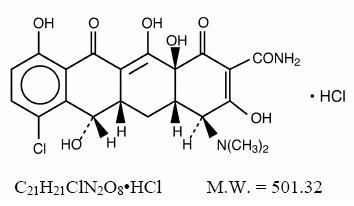
Demeclocycline hydrochloride film coated tablets for oral administration contain 150 mg or 300 mg of demeclocycline hydrochloride and the following inactive ingredients: alginic acid, corn starch, ethylcellulose, FD&C Red 40 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, talc and titanium dioxide. In addition, the 150 mg tablet contains D&C Red 27 aluminum lake.
-
CLINICAL PHARMACOLOGY
The absorption of demeclocycline is slower than that of tetracycline. The time to reach the peak concentration is about 4 hours. After a 150 mg oral dose of demeclocycline tablet, the mean concentrations at 1 hour and 3 hours are 0.46 and 1.22 mcg/mL (n = 6), respectively. The serum half-life ranges between 10 and 16 hours. Pharmacokinetics
When demeclocycline hydrochloride is given concomitantly with some dairy products, or antacids containing aluminum, calcium, or magnesium, the extent of absorption is reduced by more than 50%.
Demeclocycline hydrochloride penetrates well into various body fluids and tissues. The percent of demeclocycline hydrochloride bound to plasma protein is about 40% using a dialysis equilibrium method and 90% using an ultra-filtration method. Demeclocycline hydrochloride, like other tetracyclines, is concentrated in the liver and excreted into the bile where it is found in much higher concentrations than in the blood. The rate of demeclocycline hydrochloride renal clearance (35 mL/min/1.73 m ) is less than half that of tetracycline. Following a single 150 mg dose of demeclocycline hydrochloride in normal volunteers, 44% (n = 8) was excreted in urine and 13% and 46%, respectively, were excreted in feces in two patients within 96 hours as active drug. 2
The tetracyclines are primarily bacteriostatic and are thought to exert their antimicrobial effect by the inhibition of protein synthesis. The tetracyclines, including demeclocycline, have a similar antimicrobial spectrum of activity against a wide range of gram-negative and gram-positive organisms. Cross-resistance of these organisms to tetracyclines is common. Microbiology
Demeclocycline has been shown to be active against most strains of the following microorganisms, both and in clinical infections as described in the section. in vitroINDICATIONS AND USAGE
AEROBIC GRAM-POSITIVE MICROORGANISMS: Bacillus anthracis Listeria monocytogenes Staphylococcus aureus Streptococcus pneumoniae
AEROBIC GRAM-NEGATIVE MICROORGANISMS: Bartonella bacilliformis species Brucella Calymmatobacterium granulomatis Campylobacter fetus Francisella tularensis Haemophilus ducreyi Haemophilus influenzae Neisseria gonorrhoeae Vibrio cholerae Yersinia pestis Because many strains of the following groups of gram-negative microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are especially recommended:
species Acinetobacter Enterobacter aerogenes Escherichia coli species Klebsiella Shigella species OTHER MICROORGANISMS: Actinomyces israelii Borrelia recurrentis Chlamydia psittaci Chlamydia trachomatis species Clostridium species Entamoeba Fusobacterium fusiforme Mycoplasma pneumoniae Propionibacterium acnes Rickettsiae subspecies Treponema pallidumpallidum subspecies Treponema pallidumpertenue Ureaplasma urealyticum Susceptibility Tests
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of demeclocycline hydrochloride or tetracycline powder. The MIC values should be interpreted according to the following criteria: 1
For organisms other than species, and species: HaemophilusNeisseria gonorrhoeaeStreptococcus2
MIC (mcg/mL) Interpretation ≤ 4 Susceptible (S) 8 Intermediate (I) ≥16 Resistant (R) For and species: HaemophilusStreptococcus2
MIC (mcg/mL) Interpretation ≤ 2 Susceptible (S) 4 Intermediate (I) ≥8 Resistant (R) For : Neisseria gonorrhoeae2
MIC (mcg/mL) Interpretation ≤ 0.25 Susceptible (S) 0.5 to 1 Intermediate (I) ≥2 Resistant (R) A report of “Susceptible” indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in blood. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard demeclocycline or tetracycline powder should provide the following MIC values: 2
Microorganism MIC (mcg/mL) Microorganism MIC (mcg/mL) ATCC 25922 E.coli, 1 to 4 ATCC 49226 N. gonorrhoeae, 0.25 to 1 ATCC 29212 E. faecalis, 8 to 32 ATCC 29213 S. aureus, 0.25 to 1 ATCC 49247 H. influenzae, 4 to 32 ATCC 49619 S. pneumoniae, 0.12 to 0.5 Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30-mcg tetracycline (as a class disk) or a 30-mcg demeclocycline hydrochloride disk to test the susceptibility of microorganisms to demeclocycline. Reports from the laboratory providing results of the standard single-disk susceptibility test with either a 30-mcg tetracycline-class disk or a 30-mcg demeclocycline disk should be interpreted according to the following criteria: For organisms other than species, and species: Diffusion Techniques
2
HaemophilusNeisseria gonorrhoeaeStreptococcus2Zone Diameter (mm) Interpretation ≥ 19 Susceptible (S) 15 to 18 Intermediate (I) ≤ 14 Resistant (R) For species: Haemophilus2
Zone Diameter (mm) Interpretation ≥ 29 Susceptible (S) 26 to 28 Intermediate (I) ≤ 25 Resistant (R) For : N. gonorrhoeae2
Zone Diameter (mm) Interpretation ≥ 38 Susceptible (S) 31 to 37 Intermediate (I) ≤ 30 Resistant (R) For species: Streptococcus2
Zone Diameter (mm) Interpretation ≥ 23 Susceptible (S) 19 to 22 Intermediate (I) ≤ 18 Resistant (R) Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for demeclocycline hydrochloride or tetracycline.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-mcg demeclocycline hydrochloride disk or the 30-mcg tetracycline-class disk should provide the following zone diameters in these laboratory test quality control strains:
2Microorganism Zone Diameter (mm) , ATCC 25922 E. coli 18 to 25 ATCC 49247 H. influenzae, 14 to 22 , ATCC 49226 N. gonorrhoeae 30 to 42 , ATCC 25923 S. aureus 24 to 30 , ATCC 49619 S. pneumoniae 27 to 31 -
INDICATIONS & USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of demeclocycline hydrochloride tablets and other antibacterial drugs, demeclocycline hydrochloride tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Demeclocycline hydrochloride is indicated in the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions below:
Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by rickettsiae; Respiratory tract infections caused by ; Lymphogranuloma venereum due to ; Psittacosis (Ornithosis) due to ; Trachoma due to , although the infectious agent is not always eliminated, as judged by immunofluorescence; Inclusion conjunctivitis caused by ; Nongonococcal urethritis in adults caused by or ; Relapsing fever due to ; Chancroid caused by ; Plague due to ; Tularemia due to ; Cholera caused by ; Campylobacter fetus infections caused by ; Brucellosis due to species (in conjunction with streptomycin); Bartonellosis due to ; Granuloma inguinale caused by ; Demeclocycline hydrochloride is indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug: ; ; species; species; Respiratory tract infections caused by ; Respiratory tract and urinary tract infections caused by species. Demeclocycline hydrochloride is indicated for treatment of infections caused by the following gram-positive microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug: Upper respiratory infections caused by pneumoniae; Skin and skin structure infections caused by . (Note: Tetracyclines, including demeclocycline, are not the drugs of choice in the treatment of any type of staphylococcal infection.) When penicillin is contraindicated, tetracyclines, including demeclocycline hydrochloride, are alternative drugs in the treatment of the following infections: Uncomplicated urethritis in men due to , and for the treatment of other uncomplicated gonococcal infections; Infections in women caused by ; Syphilis caused by subspecies ; Yaws caused by subspecies ; Listeriosis due to ; Anthrax due to ; Vincent’s infection caused by ; Actinomycosis caused by ; Clostridial diseases caused by species. In acute intestinal amebiasis, demeclocycline hydrochloride may be a useful adjunct to amebicides. In severe acne, demeclocycline hydrochloride may be a useful adjunctive therapy.
Mycoplasma pneumoniae
Chlamydia trachomatis
Chlamydia psittaci
Chlamydia trachomatis
Chlamydia trachomatis
Ureaplasma urealyticumChlamydia trachomatis
Borrelia recurrentis
Haemophilus ducreyi
Yersinia pestis
Francisella tularensis
Vibrio cholerae
Campylobacter fetus
Brucella
Bartonella bacilliformis
Calymmatobacterium granulomatis
Escherichia coli
Enterobacter aerogenes
Shigella
Acinetobacter
Haemophilus influenzae
Klebsiella
Streptococcus
Staphylococcus aureus
Neisseria gonorrhoeae
Neisseria gonorrhoeae
Treponema pallidumpallidum
Treponema pallidumpertenue
Listeria monocytogenes
Bacillus anthracis
Fusobacterium fusiforme
Actinomyces israelii
Clostridium
- CONTRAINDICATIONS
-
WARNINGS
DEMECLOCYCLINE HYDROCHLORIDE, LIKE OTHER TETRACYCLINE-CLASS ANTIBIOTICS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY, OR IF THE PATIENT BECOMES PREGNANT WHILE TAKING THESE DRUGS, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS.
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN). This adverse reaction is more common during long-term use of the drugs but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED DURING TOOTH DEVELOPMENT UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED.
All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every six hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy.
The anti-anabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis.
If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulation of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated and, if therapy is prolonged, serum level determinations of the drug may be advisable.
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Phototoxic reactions can occur in individuals taking demeclocycline, and are characterized by severe burns of exposed surfaces resulting from direct exposure of patients to sunlight during therapy with moderate or large doses of demeclocycline. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur, and treatment should be discontinued at the first evidence of erythema of the skin.
Administration of demeclocycline hydrochloride has resulted in appearance of the diabetes insipidus syndrome (polyuria, polydipsia and weakness) in some patients on long-term therapy. The syndrome has been shown to be nephrogenic, dose-dependent and reversible on discontinuance of therapy.
Patients who are experiencing central nervous system symptoms associated with demeclocycline therapy, should be cautioned about driving vehicles or using hazardous machinery while on demeclocycline therapy.
-
PRECAUTIONS
Prescribing demeclocycline hydrochloride tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increase the risk of the development of drug-resistant bacteria. General
Pseudotumor cerebri (benign intracranial hypertension) in adults has been associated with the use of tetracyclines. The usual clinical manifestations are headache and blurred vision. Bulging fontanels have been associated with the use of tetracyclines in infants. While both of these conditions and related symptoms usually resolve soon after discontinuation of the tetracycline, the possibility for permanent sequelae exists.
As with other antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate therapy should be instituted.
Incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy, when indicated.
Patients should be counseled that antibacterial drugs including demeclocycline hydrochloride tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g. the common cold). When demeclocycline hydrochloride tablets are prescribed to treat bacterial infection, patients should be told that although it is common to fell better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by demeclocycline hydrochloride tablets or other antibacterial drugs in the future. Information for Patients
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema.
Concurrent use of tetracyclines with oral contraceptives may render oral contraceptives less effective. (See .) Drug Interactions
Patients should be informed that demeclocycline hydrochloride tablets should be taken at least 1 hour before meals or 2 hours after meals. (See .) DOSAGE AND ADMINISTRATION
Unused supplies of tetracycline antibiotics should be discarded by the expiration date.
Patients who are experiencing headache, dizziness, light-headedness, vertigo, or blurred vision while on demeclocycline therapy, should be cautioned about driving vehicles or using hazardous machinery while receiving demeclocycline therapy. (See .) WARNINGS
Laboratory Tests
In venereal diseases when coexistent syphilis is suspected, darkfield examination should be done before treatment is started and the blood serology repeated monthly for at least 4 months.
In long-term therapy, periodic laboratory evaluation of organ systems, including hematopoietic, renal, and hepatic, should be performed.
All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with demeclocycline hydrochloride should have a follow-up serologic test for syphilis after 3 months.
Drug Interactions
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
Since bacteriostatic drugs may interfere with the bactericidal action of penicillins, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
Concurrent use of tetracyclines with oral contraceptives may render oral contraceptives less effective.
The concurrent use of tetracyclines and methoxyflurane has been reported to result in fatal renal toxicity.
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium or magnesium, and by iron-containing preparations.
Long-term studies in animals to evaluate carcinogenic potential of demeclocycline hydrochloride have not been conducted. However, there has been evidence of oncogenic activity in rats in studies with the related antibiotics oxytetracycline (adrenal and pituitary tumors) and minocycline (thyroid tumors). Carcinogenesis, Mutagenesis, Impairment of Fertility
Although mutagenicity studies of demeclocycline hydrochloride have not been conducted, positive results in mammalian cell assays (i.e., mouse lymphoma and Chinese hamster lung cells) have been reported for related antibiotics (tetracycline hydrochloride and oxytetracycline). (See and .) in vitroWARNINGSANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGY
Demeclocycline hydrochloride had no effect on fertility when administered in the diet to male and female rats at a daily intake of 45 times the human dose.
. (See .) Pregnancy
Teratogenic effects. Pregnancy Category DWARNINGSResults of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has been noted in animals treated early in pregnancy.
(See .) Nonteratogenic effects.WARNINGS
The effect of tetracyclines on labor and delivery is unknown. Labor and Delivery
Tetracyclines are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from the tetracyclines, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. Nursing Mothers
(See .) WARNINGS
Not for use in patients younger than eight years of age. See , ( subsection) and . Pediatric Use
WARNINGSPRECAUTIONSGeneralDOSAGE AND ADMINISTRATION -
ADVERSE REACTIONS
The following reactions have been reported in patients receiving tetracyclines:
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, pancreatitis, and inflammatory lesions (with monilial overgrowth) in the anogenital region, increases in liver enzymes, and hepatic toxicity has been reported rarely.
Rarely, hepatitis and liver failure have been reported. These reactions have been caused by both the oral and parenteral administration of tetracyclines.
Instances of esophageal ulcerations have been reported in patients receiving oral tetracyclines. Most of the patients were reported to have taken the medication immediately before lying down. (See .) DOSAGE AND ADMINISTRATION
Skin: Maculopapular and erythematous rashes, erythema multiforme. Exfoliative dermatitis has been reported but is uncommon. Fixed drug eruptions and Stevens-Johnson syndrome have been reported rarely. Lesions occurring on the glans penis have caused balanitis. Pigmentation of the skin and mucous membranes has also been reported. Photosensitivity is discussed above. (See .) WARNINGS
Renal toxicity: Acute renal failure. Rise in BUN has been reported and is apparently dose related. Nephrogenic diabetes insipidus. (See .) WARNINGS
Hypersensitivity reactions: Urticaria, angioneurotic edema, polyarthralgia, anaphylaxis, anaphylactoid purpura, pericarditis, exacerbation of systemic lupus erythematosus, lupus-like syndrome, pulmonary infiltrates with eosinophilia.
Hematologic: Hemolytic anemia, thrombocytopenia, neutropenia and eosinophilia have been reported.
CNS: Pseudotumor cerebri (benign intracranial hypertension) in adults and bulging fontanels in infants (see ). Dizziness, headache, tinnitus, and visual disturbances have been reported. Myasthenic syndrome has been reported rarely. PRECAUTIONS-General
Other: When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function studies are known to occur. Very rare cases of abnormal thyroid function have been reported.
Tooth discoloration has occurred in pediatric patients less than 8 years of age (see ), and also has been reported rarely in adults. WARNINGS
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
Therapy should be continued for at least 24 to 48 hours after symptoms and fever have subsided.
Concomitant therapy: Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium, and by iron-containing preparations. Foods and some dairy products also interfere with absorption. Oral forms of tetracycline should be given at least 1 hour before or 2 hours after meals.
In patients with renal impairment: (See .) Tetracyclines should be used cautiously in patients with impaired renal function. Total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses. WARNINGS
In patients with liver impairment: Tetracyclines should be used cautiously in patients with impaired liver function. Total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses.
Administration of adequate amounts of fluid with the oral formulations of tetracyclines is recommended to wash down the drugs and reduce the risk of esophageal irritation and ulceration. (See .) ADVERSE REACTIONS
Adults: Usual daily dose - Four divided doses of 150 mg each or two divided doses of 300 mg each.
For pediatric patients above eight years of age: Usual daily dose, 7 to 13 mg per kg body weight per day, depending upon the severity of the disease, divided into two to four doses not to exceed adult dosage of 600 mg per day.
Gonorrhea patients sensitive to penicillin may be treated with demeclocycline administered as an initial oral dose of 600 mg followed by 300 mg every 12 hours for four days to a total of 3 grams.
- HOW SUPPLIED
- DEMECLOCYCLINE HYDROCHLORIDE TABLET
-
INGREDIENTS AND APPEARANCE
DEMECLOCYCLINE HYDROCHLORIDE
demeclocycline hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68151-0155(NDC:65162-554) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEMECLOCYCLINE HYDROCHLORIDE (UNII: 29O079NTYT) (DEMECLOCYCLINE - UNII:5R5W9ICI6O) DEMECLOCYCLINE HYDROCHLORIDE 150 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) STARCH, CORN (UNII: O8232NY3SJ) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color RED Score no score Shape ROUND Size 9mm Flavor Imprint Code AN;54 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-0155-1 1 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065425 04/01/2010 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-0155)


