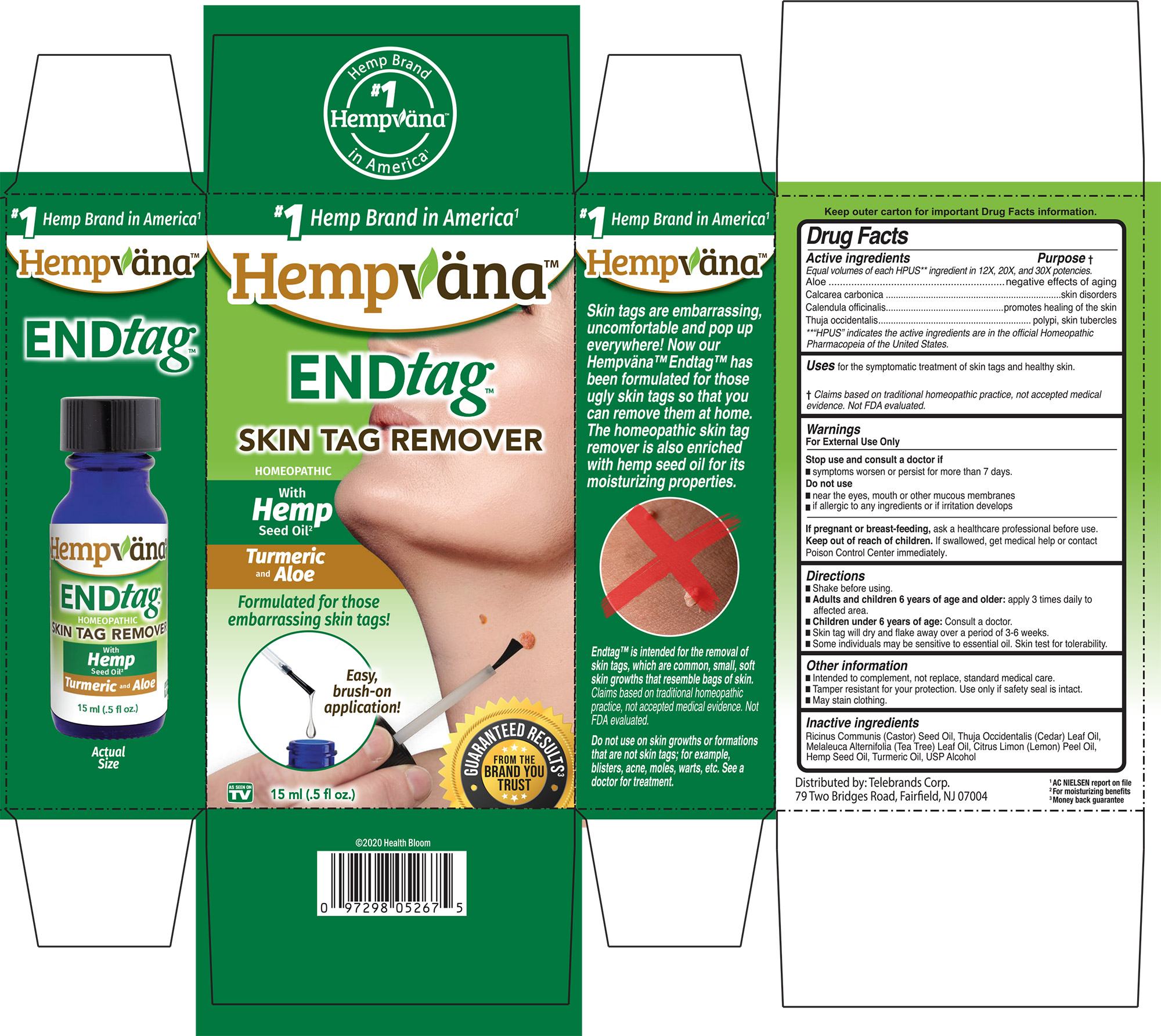
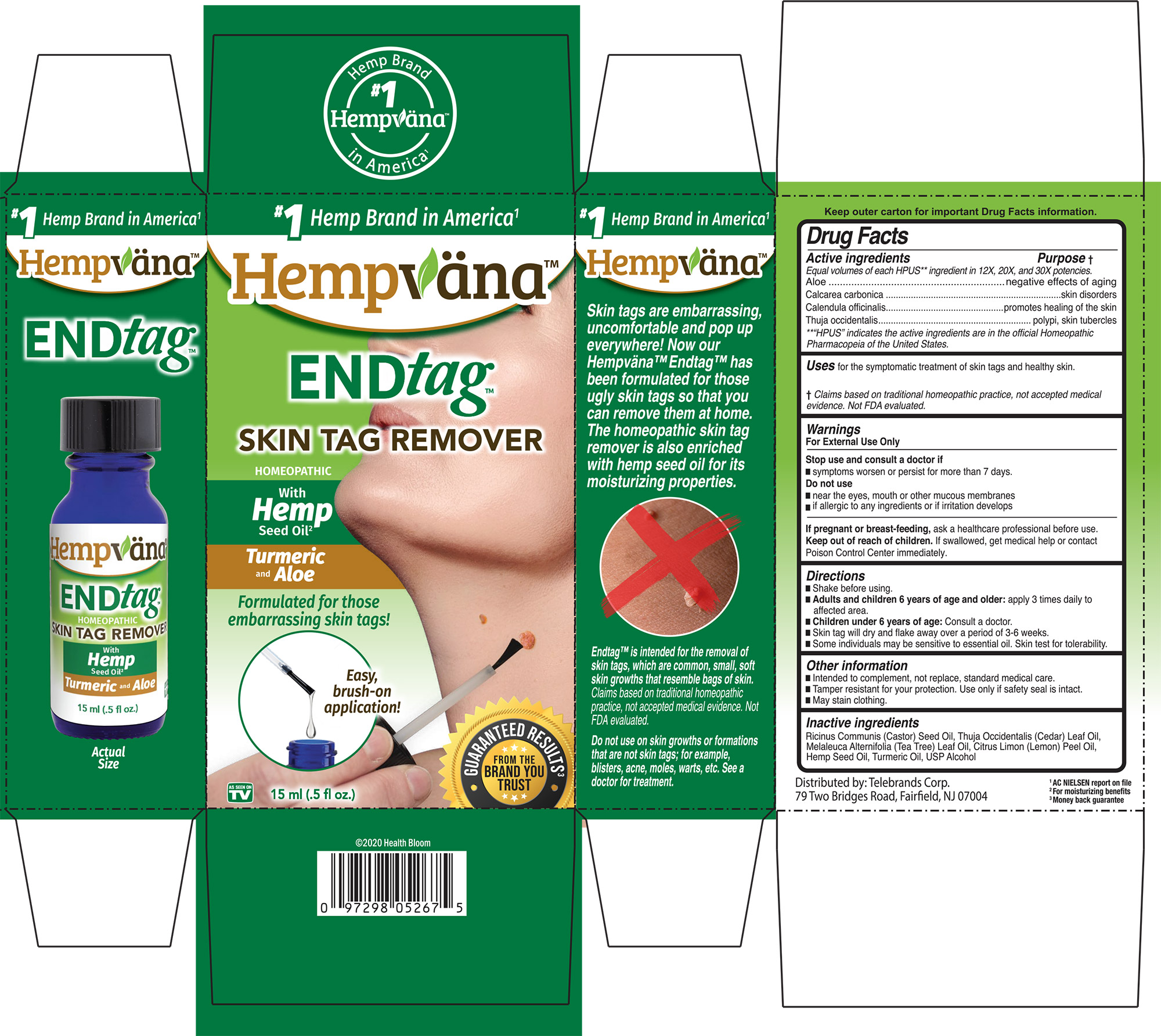
Label: ENDTAG- aloe, calcarea carbonica, calendula officinalis, thuja occidentalis liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-9650-0 - Packager: King Bio Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HPUS Active Ingredients
-
PURPOSE
Active ingredients ................. Purpose
Equal volumes of each HPUS** ingredient in 12X, 20X, and 30X potencies.
Aloe...........................................negative effects of againg
Calcarea carbonica...............skin disorders
Calendula officinalis.............promotes healing of the skin
Thuja occidentalis.................polypi, skin, tubercles
**“HPUS” indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ENDTAG
aloe, calcarea carbonica, calendula officinalis, thuja occidentalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-9650 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 12 [hp_X] in 15 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 15 mL ALOE SUCCOTRINA WHOLE (UNII: M6U8N4MD5P) (ALOE SUCCOTRINA WHOLE - UNII:M6U8N4MD5P) ALOE SUCCOTRINA WHOLE 12 [hp_X] in 15 mL THUJA OCCIDENTALIS WHOLE (UNII: 5HBV6WCE3N) (THUJA OCCIDENTALIS WHOLE - UNII:5HBV6WCE3N) THUJA OCCIDENTALIS WHOLE 12 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength CEDAR LEAF OIL (UNII: BJ169U4NLG) ALCOHOL (UNII: 3K9958V90M) TURMERIC OIL (UNII: 6KGS8SP16U) TEA TREE OIL (UNII: VIF565UC2G) LEMON OIL (UNII: I9GRO824LL) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CASTOR OIL (UNII: D5340Y2I9G) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-9650-0 1 in 1 CARTON 06/15/2020 1 59 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 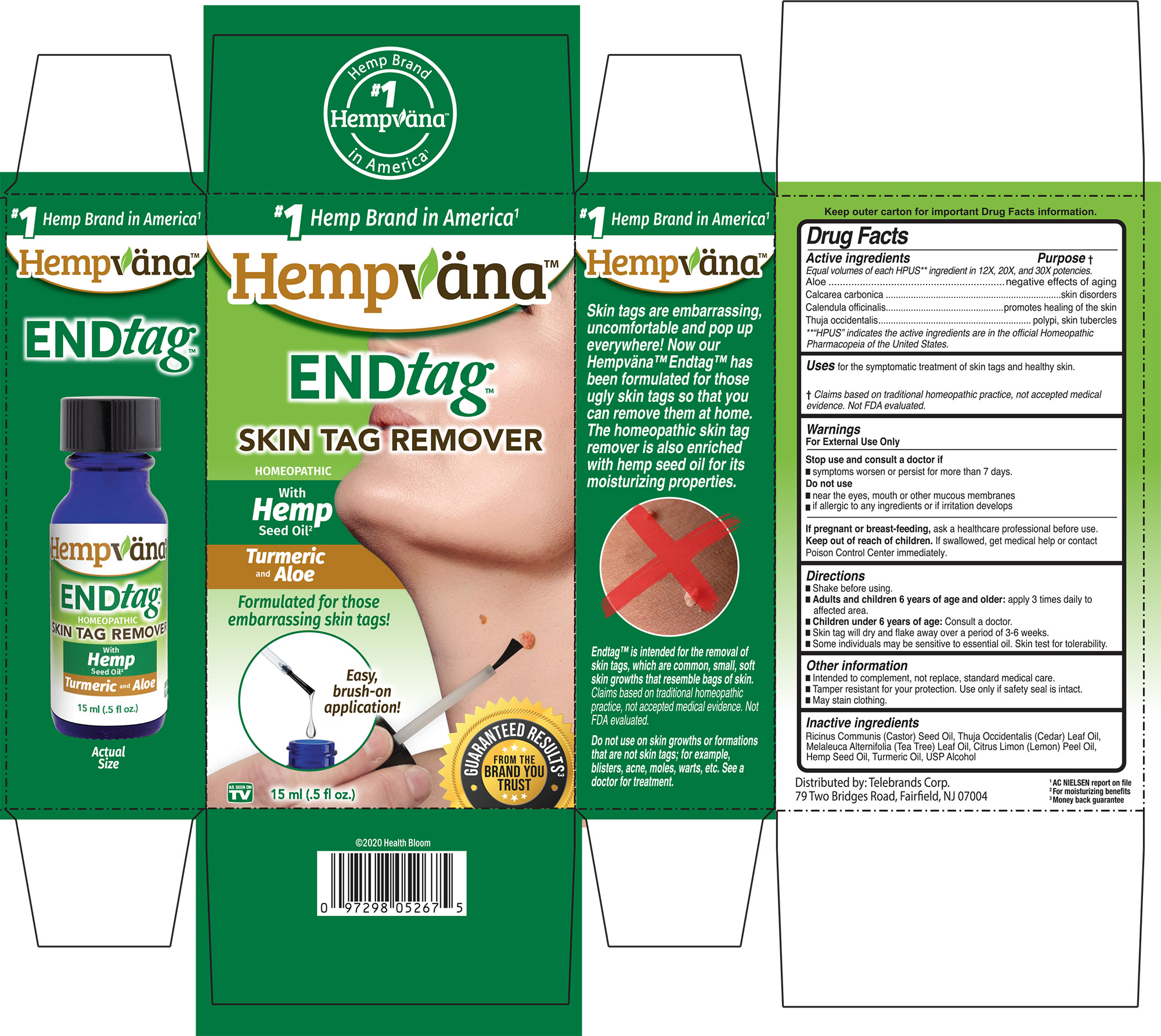
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/15/2020 Labeler - King Bio Inc (617901350) Registrant - King Bio Inc (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc 617901350 manufacture(57955-9650) , api manufacture(57955-9650)