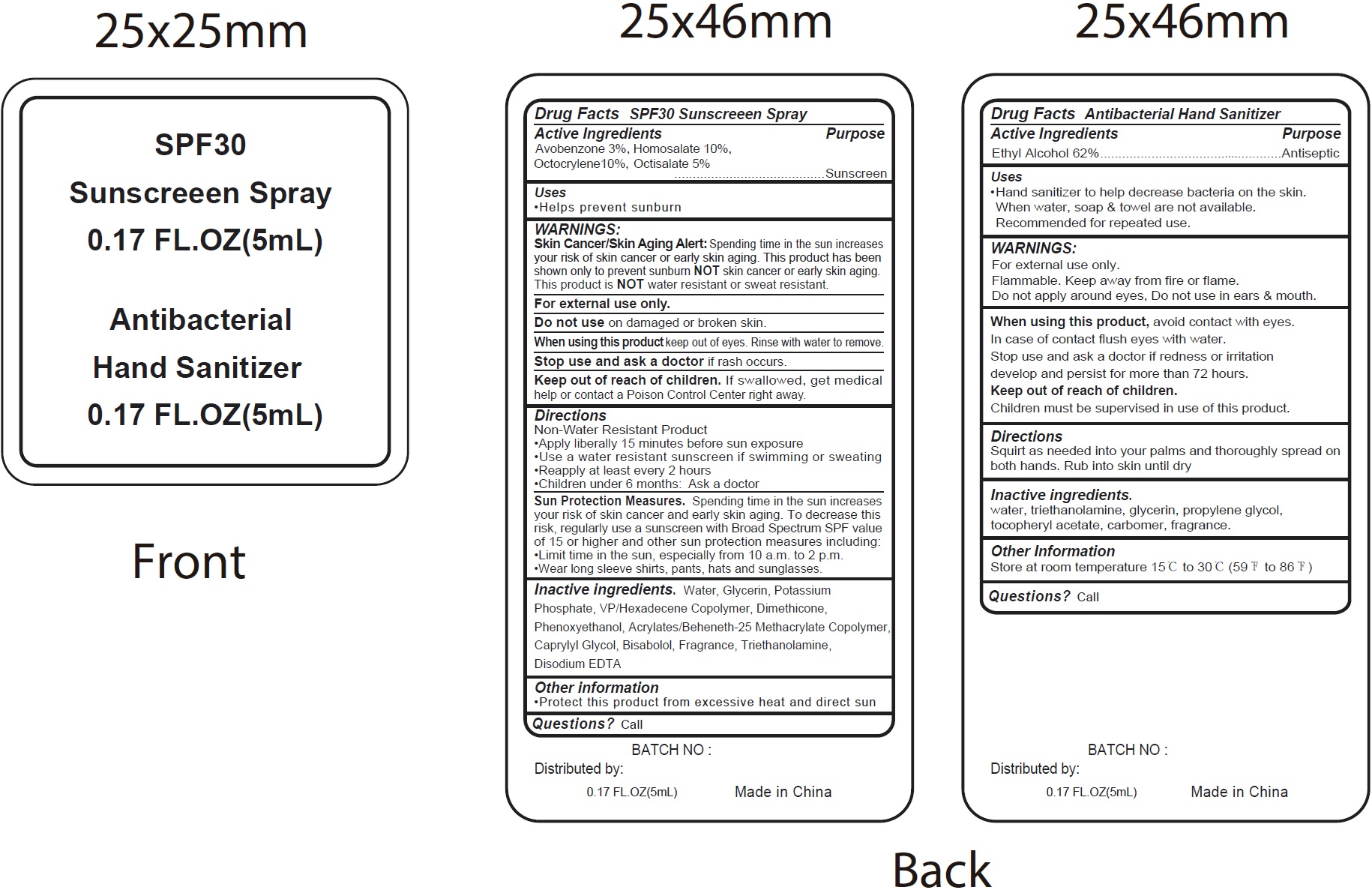
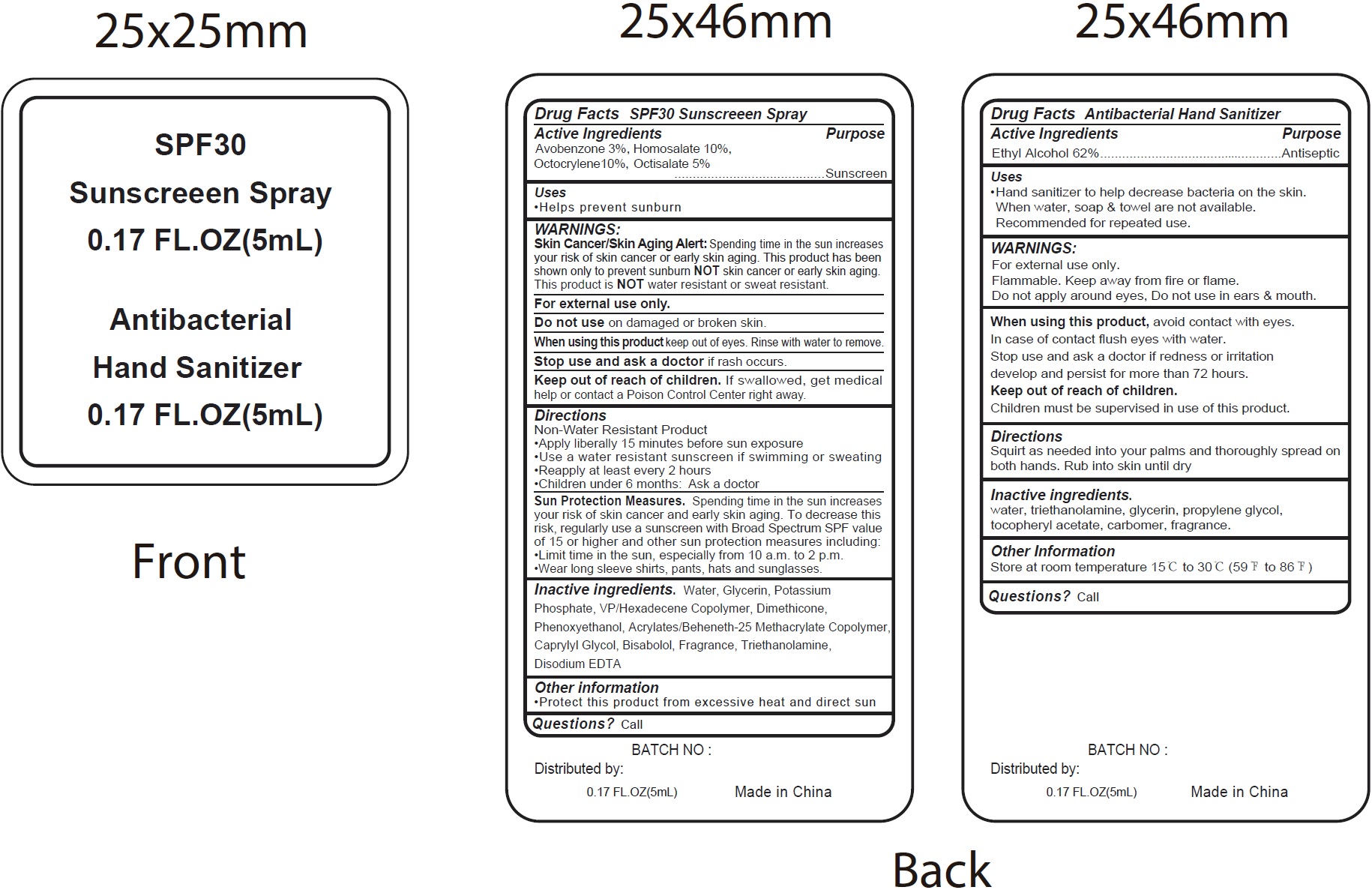
Label: SPF30 SUNSCREEN AND ANTIBACTERIAL HAND SANITIZER- alcohol, avobenzone, homosalate, octocrylene, octisalate kit
- NDC Code(s): 70412-119-05, 70412-238-05, 70412-817-05
- Packager: Zhejiang Ayan Biotech Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Antibacterial Hand Sanitizer
- Active Ingredients
- Uses
- WARNINGS:
- Directions
- Inactive ingredients.
- Other Information
- Questions?
- Drug Facts SPF30 Sunscreeen Spray
- Active Ingredients
- Uses
- WARNINGS:
-
Directions
Non-Water Resistant Product • Apply liberally 15 minutes before sun exposure • Use a water resistant sunscreen if swimming or sweating • Reapply at least every 2 hours • Children under 6 months: Ask a doctor
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including: •Limit time in the sun, especially from 10 a.m. to 2 p.m. •Wear long sleeve shirts, pants, hats and sunglasses. Sun Protection Measures.
- Inactive ingredients.
- Other information
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SPF30 SUNSCREEN AND ANTIBACTERIAL HAND SANITIZER
alcohol, avobenzone, homosalate, octocrylene, octisalate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70412-817 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70412-817-05 1 in 1 KIT 09/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 5 mL Part 2 1 TUBE 5 mL Part 1 of 2 ANTIBACTERIAL HAND SANITIZER
ethyl alcohol sprayProduct Information Item Code (Source) NDC:70412-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 620 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70412-119-05 1 in 1 BOX 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/01/2018 Part 2 of 2 SPF30 SUNSCREEN
avobenzone, homosalate, octocrylene, ocitsalate sprayProduct Information Item Code (Source) NDC:70412-238 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) LEVOMENOL (UNII: 24WE03BX2T) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70412-238-05 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2018 Labeler - Zhejiang Ayan Biotech Co., Ltd. (544377996) Establishment Name Address ID/FEI Business Operations Zhejiang Ayan Biotech Co., Ltd. 544377996 manufacture(70412-817, 70412-119, 70412-238)