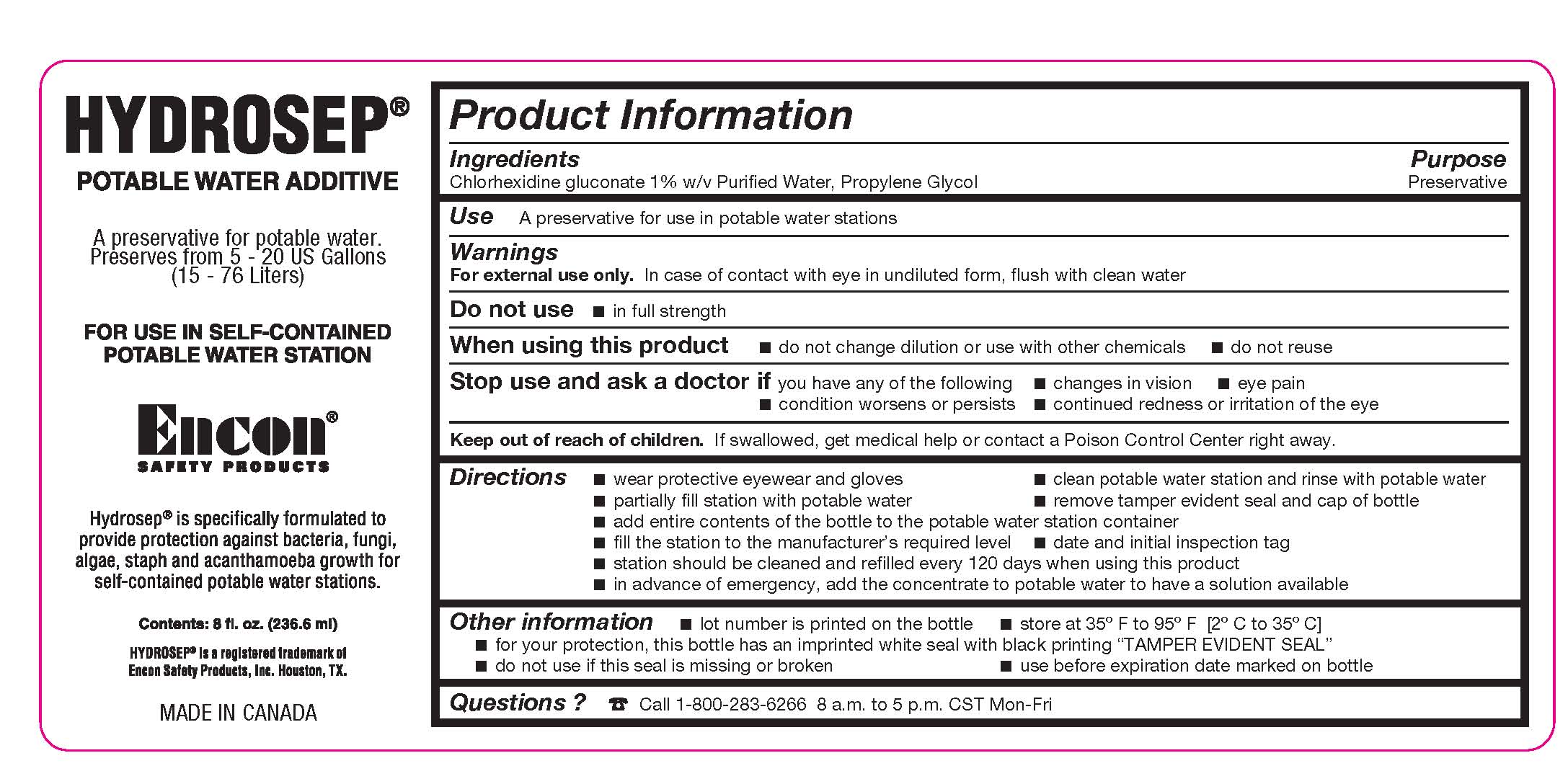
Label: EYEWASH STATION ADDITIVE CONCENTRATE- chlorhexidine gluconate and propylene glycol liquid
- NDC Code(s): 70290-120-01, 70290-120-05, 70290-120-06
- Packager: Encon Safety Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Ingredients
- Purpose
- Use
- Warnings
-
Directions
- wear protective eyewear and gloves
- clean potable eyewash station and rinse with potable water
- partially fill station with potable water
- remove tamper evident seal and cap of bottle
- add entire contents of the bottle to the eyewash station container
- fill the station to the manufacturer's required level
- date and initial inspection tag
- station should be cleaned and refilled every 120 days when using this product
- in advance of emergency, add the concentrate to potable water to have a solution available
- Other information
- Questions ?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
EYEWASH STATION ADDITIVE CONCENTRATE
chlorhexidine gluconate and propylene glycol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70290-120 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 145.6 kg in 2800 L PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 280 kg in 2800 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2374.4 L in 2800 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70290-120-01 0.237 L in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 12/02/2020 2 NDC:70290-120-05 0.946 L in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 12/02/2020 3 NDC:70290-120-06 3.786 L in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 12/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 12/02/2020 Labeler - Encon Safety Products, Inc. (008102410) Registrant - Niagara Pharmaceuticals Inc. (205477792) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals Inc. 205477792 manufacture(70290-120)