Label: PHENTERMINE HCL tablet
- NDC Code(s): 80425-0118-1
- Packager: Advanced Rx Pharmacy of Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 0527-1445
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Indications and Usage Section
1 INDICATIONS AND USAGE
Phentermine hydrochloride tablets are indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index ≥ 30 kg/m2, or ≥ 27 kg/m2 in the presence of other risk factors (e.g., controlled hypertension, diabetes, hyperlipidemia).
Below is a chart of body mass index (BMI) based on various heights and weights.
BMI is calculated by taking the patient’s weight, in kilograms (kg), divided by the patient’s height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
BODY MASS INDEX (BMI), kg/m2
Height (feet, inches)
Weight
(pounds) 5'0" 5'3" 5'6" 5'9" 6'0" 6'3"
140 27 25 23 21 19 18
150 29 27 24 22 20 19
160 31 28 26 24 22 20
170 33 30 28 25 23 21
180 35 32 29 27 25 23
190 37 34 31 28 26 24
200 39 36 32 30 27 25
210 41 37 34 31 29 26
220 43 39 36 33 30 28
230 45 41 37 34 31 29
240 47 43 39 36 33 30
250 49 44 40 37 34 31The limited usefulness of agents of this class, including phentermine, [see Clinical Pharmacology (12.1, 12.2)] should be measured against possible risk factors inherent in their use such as those described below.
-
Dosage and Administration Section
2 DOSAGE AND ADMINISTRATION
2.1 Exogenous Obesity
Dosage should be individualized to obtain an adequate response with the lowest effective dose.
The usual adult dose is one tablet (37.5 mg) daily, as prescribed by the physician, administered before breakfast or 1 to 2 hours after breakfast. The dosage may be adjusted to the patient’s need. For some patients, half tablet (18.75 mg) daily may be adequate, while in some cases it may be desirable to give half tablets (18.75 mg) two times a day. Phentermineis not recommended for use in pediatric patients ≤ 16 years of age.
Late evening medication should be avoided because of the possibility of resulting insomnia.
2.2 Dosage in Patients With Renal Impairment
The recommended maximum dosage of phentermine is 15 mg daily for patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2). Avoid use of phentermine in patients with eGFR less than 15 mL/min/1.73m2 or end-stage renal disease requiring dialysis [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- Dosage Forms and Strengths
-
Contraindications
- History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension)
- During or within 14 days following the administration of monoamine oxidase inhibitors
- Hyperthyroidism
- Glaucoma
- Agitated states
- History of drug abuse
- Pregnancy [see Use in Specific Populations ( 8.1)]
- Nursing [see Use in Specific Populations ( 8.3)]
- Known hypersensitivity, or idiosyncrasy to the sympathomimetic amines
-
Warnings and Precautions
5.1 Coadministration With Other Drug Products for Weight Loss
Phentermine hydrochloride tablets are indicated only as short-term (a few weeks) monotherapy for the management of exogenous obesity. The safety and efficacy of combination therapy with phentermine and any other drug products for weight loss including prescribed drugs, over-the-counter preparations, and herbal products, or serotonergic agents such as selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline, fluvoxamine, paroxetine), have not been established. Therefore, coadministration of phentermine and these drug products is not recommended.
5.2 Primary Pulmonary Hypertension
Primary Pulmonary Hypertension (PPH) - a rare, frequently fatal disease of the lungs - has been reported to occur in patients receiving a combination of phentermine with fenfluramine or dexfenfluramine. The possibility of an association between PPH and the use of phentermine alone cannot be ruled out; there have been rare cases of PPH in patients who reportedly have taken phentermine alone. The initial symptom of PPH is usually dyspnea. Other initial symptoms may include angina pectoris, syncope or lower extremity edema. Patients should be advised to report immediately any deterioration in exercise tolerance. Treatment should be discontinued in patients who develop new, unexplained symptoms of dyspnea, angina pectoris, syncope or lower extremity edema, and patients should be evaluated for the possible presence of pulmonary hypertension.
5.3 Valvular Heart Disease
Serious regurgitant cardiac valvular disease, primarily affecting the mitral, aortic and/or tricuspid valves, has been reported in otherwise healthy persons who had taken a combination of phentermine with fenfluramine or dexfenfluramine for weight loss. The possible role of phentermine in the etiology of these valvulopathies has not been established and their course in individuals after the drugs are stopped is not known. The possibility of an association between valvular heart disease and the use of phentermine alone cannot be ruled out; there have been rare cases of valvular heart disease in patients who reportedly have taken phentermine alone.
5.4 Development of Tolerance, Discontinuation in Case of Tolerance
When tolerance to the anorectant effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.
5.5 Effect on the Ability to Engage in Potentially Hazardous Tasks
Phentermine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.
5.6 Risk of Abuse and Dependence
Phentermine is related chemically and pharmacologically to amphetamine (d- and dll-amphetamine) and other related stimulant drugs that have been extensively abused. The possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. See Drug Abuse and Dependence (9) and Overdosage (10).
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
5.7 Usage With Alcohol
Concomitant use of alcohol with phentermine may result in an adverse drug reaction.
5.8 Use in Patients With Hypertension
Use caution in prescribing phentermine for patients with even mild hypertension (risk of increase in blood pressure).
5.9 Use in Patients on Insulin or Oral Hypoglycemic Medications for Diabetes Mellitus
A reduction in insulin or oral hypoglycemic medications in patients with diabetes mellitus may be required.
-
Adverse Reactions
The following adverse reactions are described, or described in greater detail, in other sections:
- Primary pulmonary hypertension [see Warnings and Precautions ( 5.2)]
- Valvular heart disease [see Warnings and Precautions ( 5.3)]
- Effect on the ability to engage in potentially hazardous tasks [see Warnings and Precautions ( 5.5)]
- Withdrawal effects following prolonged high dosage administration [see Drug Abuse and Dependence ( 9.3)]The following adverse reactions to phentermine have been identified:
Cardiovascular
Primary pulmonary hypertension and/or regurgitant cardiac valvular disease, palpitation, tachycardia, elevation of blood pressure, ischemic events.Central Nervous System
Overstimulation, restlessness, dizziness, insomnia, euphoria, dysphoria, tremor, headache, psychosis.Gastrointestinal
Dryness of the mouth, unpleasant taste, diarrhea, constipation, other gastrointestinal disturbances.Allergic
Urticaria.Endocrine
Impotence, changes in libido. -
Drug Interactions
7.1 Monoamine Oxidase Inhibitors
Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive crisis.
7.2 Alcohol
Concomitant use of alcohol with phentermine may result in an adverse drug reaction.
7.3 Insulin and Oral Hypoglycemic Medications
Requirements may be altered [see Warnings and Precautions (5.9)].
7.4 Adrenergic Neuron Blocking Drugs
Phentermine may decrease the hypotensive effect of adrenergic neuron blocking drugs.
-
Use in Specific Populations
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category X
Phentermine is contraindicated during pregnancy because weight loss offers no potential benefit to a pregnant woman and may result in fetal harm. A minimum weight gain, and no weight loss, is currently recommended for all pregnant women, including those who are already overweight or obese, due to obligatory weight gain that occurs in maternal tissues during pregnancy. Phentermine has pharmacologic activity similar to amphetamine (d- and dll-amphetamine) [see Clinical Pharmacology (12.1)]. Animal reproduction studies have not been conducted with phentermine. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.8.3 Nursing Mothers
It is not known if phentermine is excreted in human milk; however, other amphetamines are present in human milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Because pediatric obesity is a chronic condition requiring long-term treatment, the use of this product, approved for short-term therapy, is not recommended.
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.8.6 Renal Impairment
Based on the reported excretion of phentermine in urine, exposure increases can be expected in patients with renal impairment [see Clinical Pharmacology (12.3)].
Use caution when administering phentermine to patients with renal impairment. In patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2), limit the dosage of phentermine to 15 mg daily [see Dosage and Administration (2.2)]. Phentermine has not been studied in patients with eGFR less than 15 mL/min/1.73m2, including end-stage renal disease requiring dialysis; avoid use in these populations.
-
Drug Abuse and Dependence
9.1 Controlled Substance
Phentermine is a Schedule IV controlled substance.
9.2 Abuse
Phentermine is related chemically and pharmacologically to the amphetamines. Amphetamines and other stimulant drugs have been extensively abused and the possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program.
9.3 Dependence
Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage of these drugs to many times than recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. A severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
-
Overdosage Section
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
10.1 Acute Overdosage
Manifestations of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmia, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Overdosage of pharmacologically similar compounds has resulted in fatal poisoning usually terminates in convulsions and coma.
Management of acute phentermine hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard. Acidification of the urine increases phentermine excretion. Intravenous phentolamine (Regitine®, CIBA) has been suggested on pharmacologic grounds for possible acute, severe hypertension, if this complicates overdosage.
10.2 Chronic Intoxication
Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxications is psychosis, often clinically indistinguishable from schizophrenia. See Drug Abuse and Dependence (9.3).
-
Description
Phentermine Hydrochloride Tablet, USP is a sympathomimetic amine anorectic. Its chemical name is α,α,-dimethylphenethylamine hydrochloride. The structural formula is as follows:
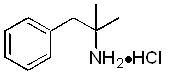
C10H15N • HCl M.W. 185.7Phentermine hydrochloride is a white, odorless, hygroscopic, crystalline powder which is soluble in water and lower alcohols, slightly soluble in chloroform and insoluble in ether.
Phentermine Hydrochloride Tablets, USP are available as oral tablets containing 37.5 mg of phentermine hydrochloride (equivalent to 30 mg of phentermine base). Each phentermine hydrochloride tablet also contains the following inactive ingredients: corn starch, lactose (anhydrous), magnesium stearate, microcrystalline, cellulose, pregelatinized starch, compressible sugar, and FD&C Blue # 1 aluminum lake.
-
Clinical Pharmacology
12.1 Mechanism of Action
Phentermine is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, amphetamine (d- and dll-amphetamine). Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics." It has not been established that the primary action of such drugs in treating obesity is one of appetite suppression since other central nervous system actions, or metabolic effects, may also be involved.
12.2 Pharmacodynamics
Typical of amphetamines include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
12.3 Pharmacokinetics
Following the administration of phentermine, phentermine reaches peak concentrations (Cmax) after 3 to 4.4 hours.
Drug Interactions
In a single-dose study comparing the exposures after oral administration of a combination capsule of 15 mg phentermine and 92 mg topiramate to the exposures after oral administration of a 15 mg phentermine capsule or a 92 mg topiramate capsule, there is no significant topiramate exposure change in the presence of phentermine. However in the presence of topiramate, phentermine Cmax and AUC increase 13% and 42%, respectively.
Specific Populations
Renal Impairment
Cumulative urinary excretion of phentermine under uncontrolled urinary pH conditions was 62% to 85%.
Systemic exposure of phentermine may increase up to 91%, 45%, and 22% in patients with severe, moderate, and mild renal impairment, respectively [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
- Non Clinical Toxicology
-
Clinical Studies
No clinical studies have been conducted with phentermine.
In relatively short-term clinical trials, adult obese subjects instructed in dietary management and treated with “anorectic” drugs lost more weight on the average than those treated with placebo and diet.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an “anorectic” drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drugs prescribed, such as the physician-investigator, the population treated and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured over several years, whereas the studies cited are restricted to a few weeks’ duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
-
How Supplied/Storage and Handling
Available as tablets containing 37.5 mg phentermine hydrochloride (equivalent to 30 mg phentermine base). Each blue speckled, capsule shaped, bisected tablet is debossed with “LCI” and “1445” on one side and plain on the other side.
Phentermine Hydrochloride Tablets, USP 37.5 mg are packaged in the following:
- Bottles of 28 Tablets NDC:80425-0118-01
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Keep out of the reach of children.
-
Patient Counseling Information
Patients must be informed that phentermine hydrochloride is a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity, and that coadministration of phentermine with other drugs for weight loss is not recommended [see Indications and Usage (1) and Warnings and Precautions (5.1)].
Patients must be instructed on how much phentermine to take, and when and how to take it [see Dosage and Administration (3)].
Advise pregnant women and nursing mothers not to use phentermine [see Use in Specific Populations (8.1, 8.3)].
Patients must be informed about the risks of use of phentermine (including the risks discussed in Warnings and Precautions), about the symptoms of potential adverse reactions and when to contact a physician and/or take other action. The risks include, but are not limited to:
Development of primary pulmonary hypertension [see Warnings and Precautions (5.2)]
Development of serious valvular heart disease [see Warnings and Precautions (5.3)]
Effects on the ability to engage in potentially hazardous tasks [see Warnings and Precautions (5.5)]
The risk of an increase in blood pressure [see Warnings and Precautions (5.8) and Adverse Reactions (6)]
The risk of interactions [see Contraindications (4), Warnings and Precautions (5.7, 5.9) and Drug Interactions (7)]See also, for example, Adverse Reactions (6) and Use in Specific Populations (8).
The patients must also be informed about
the potential for developing tolerance and actions if they suspect development of tolerance [see Warnings and Precautions (5.4)] and
the risk of dependence and the potential consequences of abuse [see Warnings and Precautions (5.6), Drug Abuse and Dependence (9), and Overdosage (10)].Tell patients to keep phentermine in a safe place to prevent theft, accidental overdose, misuse or abuse. Selling or giving away phentermine may harm others and is against the law.
Distributed by:
Lannett Company, Inc.
Philadelphia, PA 19136
Distributed By:
Advanced Rx Pharmacy of Tennessee, LLC
Nashville, TN 37211
CIB70550D
Rev. 11/2020
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PHENTERMINE HCL
phentermine hcl tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80425-0118(NDC:0527-1445) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENTERMINE HYDROCHLORIDE (UNII: 0K2I505OTV) (PHENTERMINE - UNII:C045TQL4WP) PHENTERMINE HYDROCHLORIDE 37.5 mg Product Characteristics Color blue Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code LCI;1445 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80425-0118-1 28 in 1 BOTTLE; Type 0: Not a Combination Product 04/15/2005 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040555 04/15/2005 Labeler - Advanced Rx Pharmacy of Tennessee, LLC (117023142) Establishment Name Address ID/FEI Business Operations Advanced Rx Pharmacy of Tennessee, LLC 117023142 repack(80425-0118)


