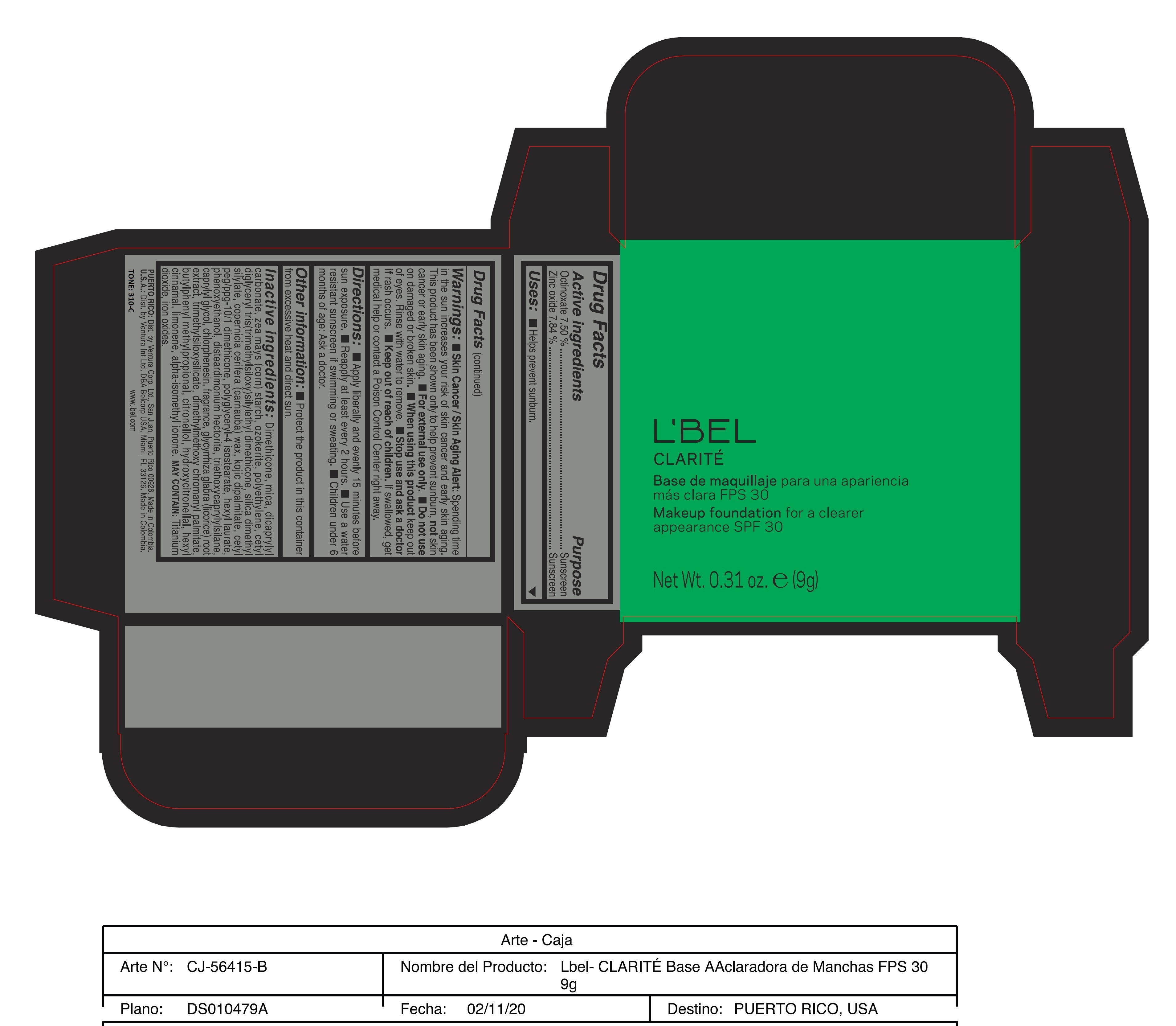
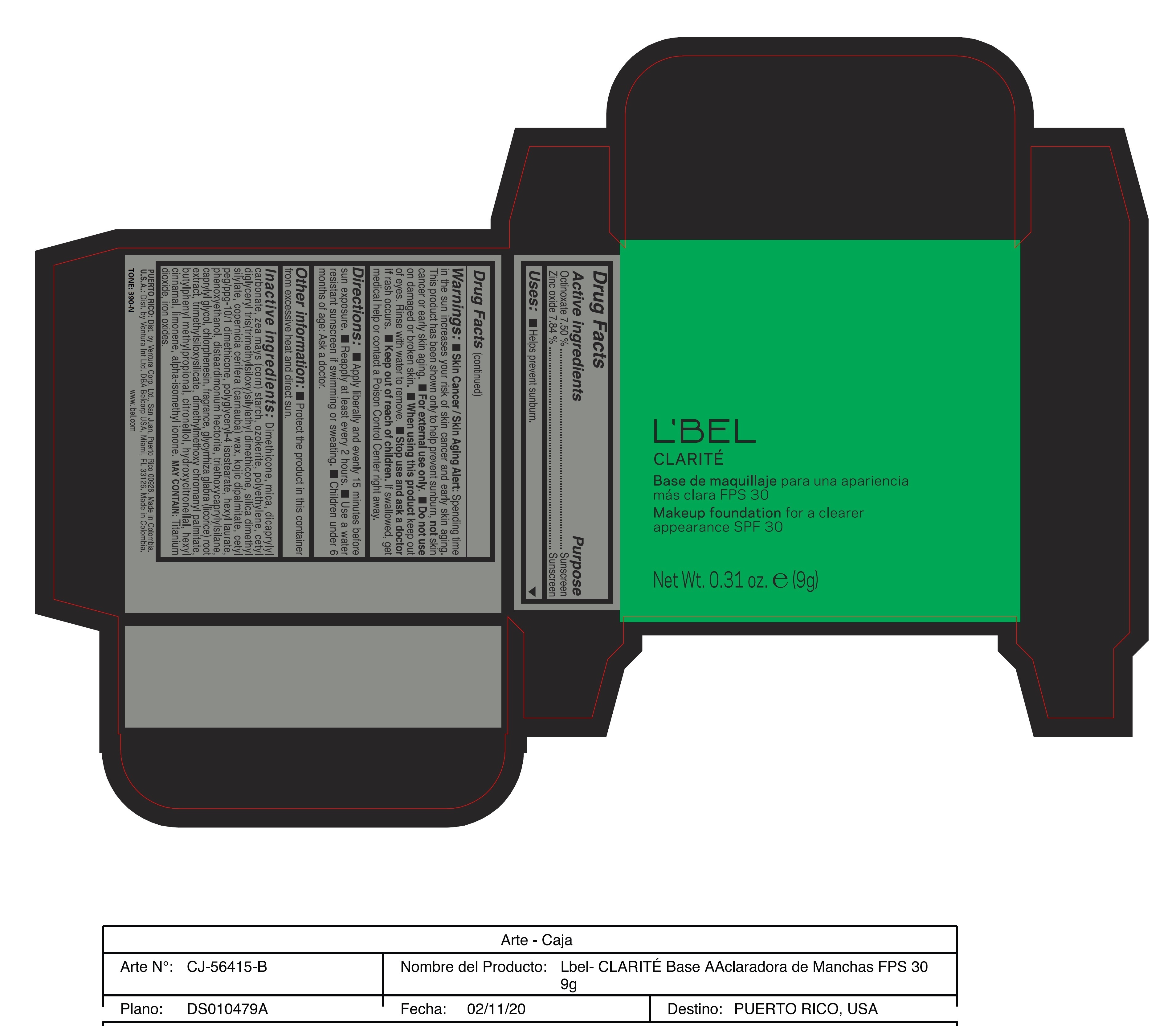
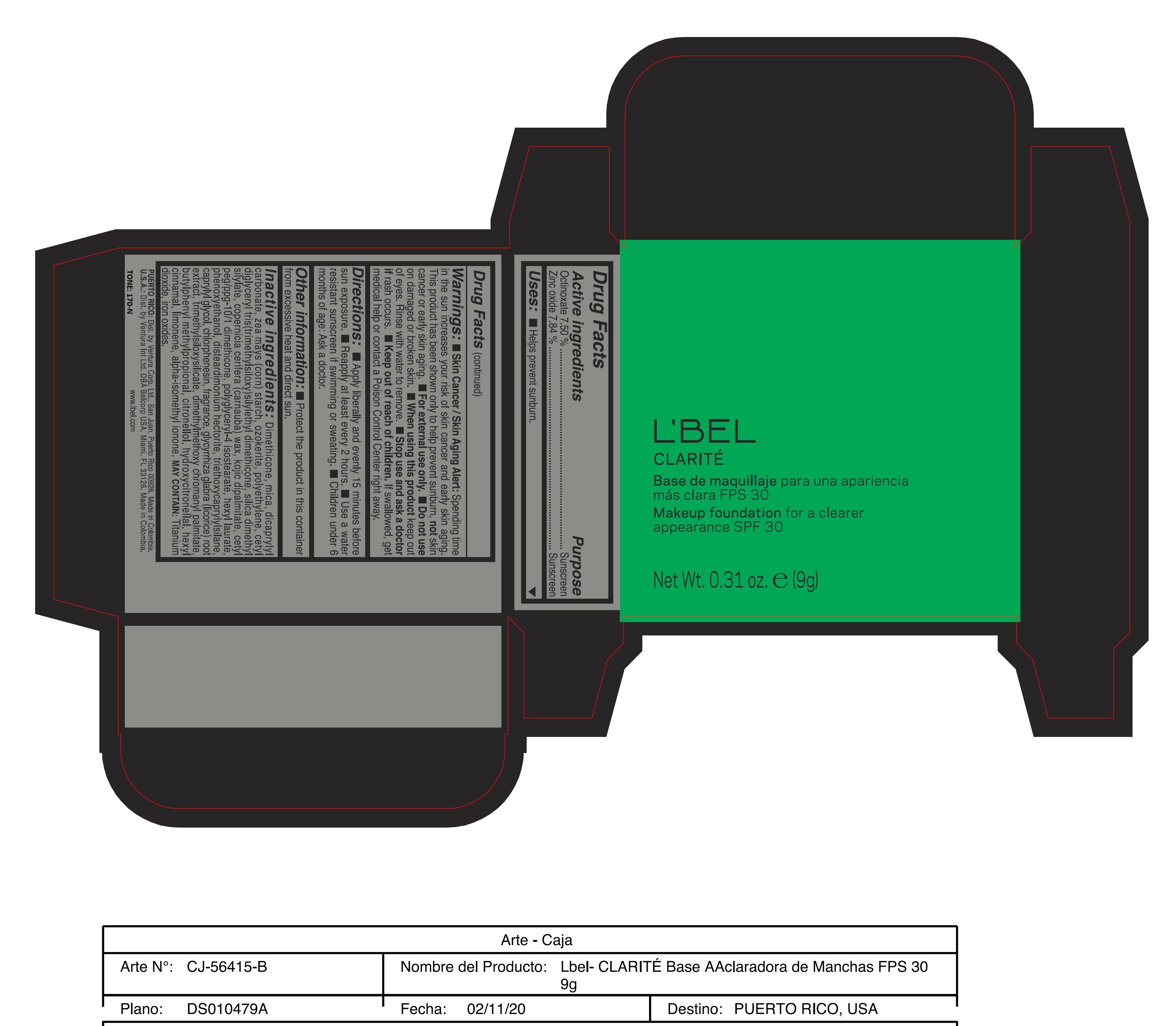
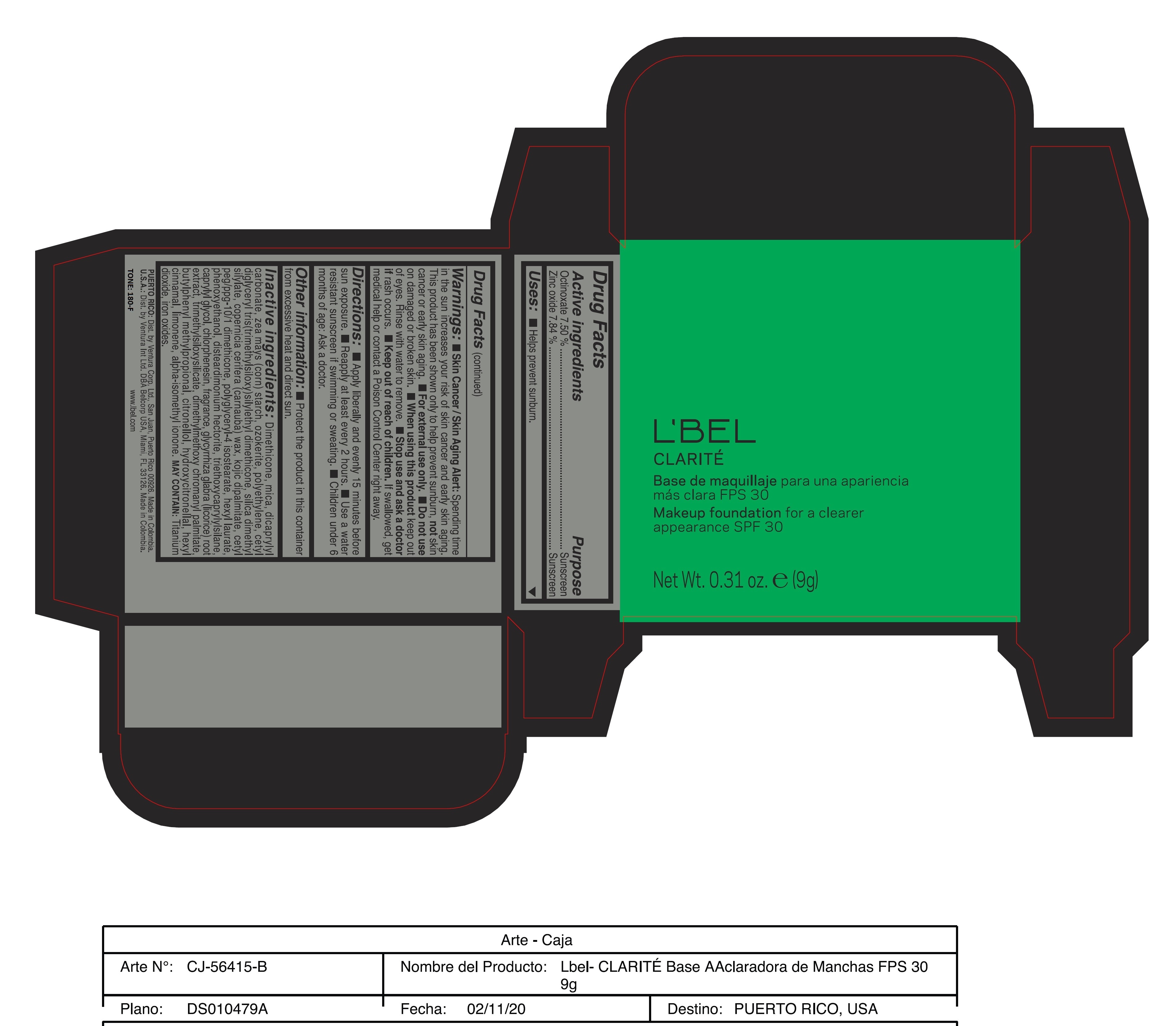
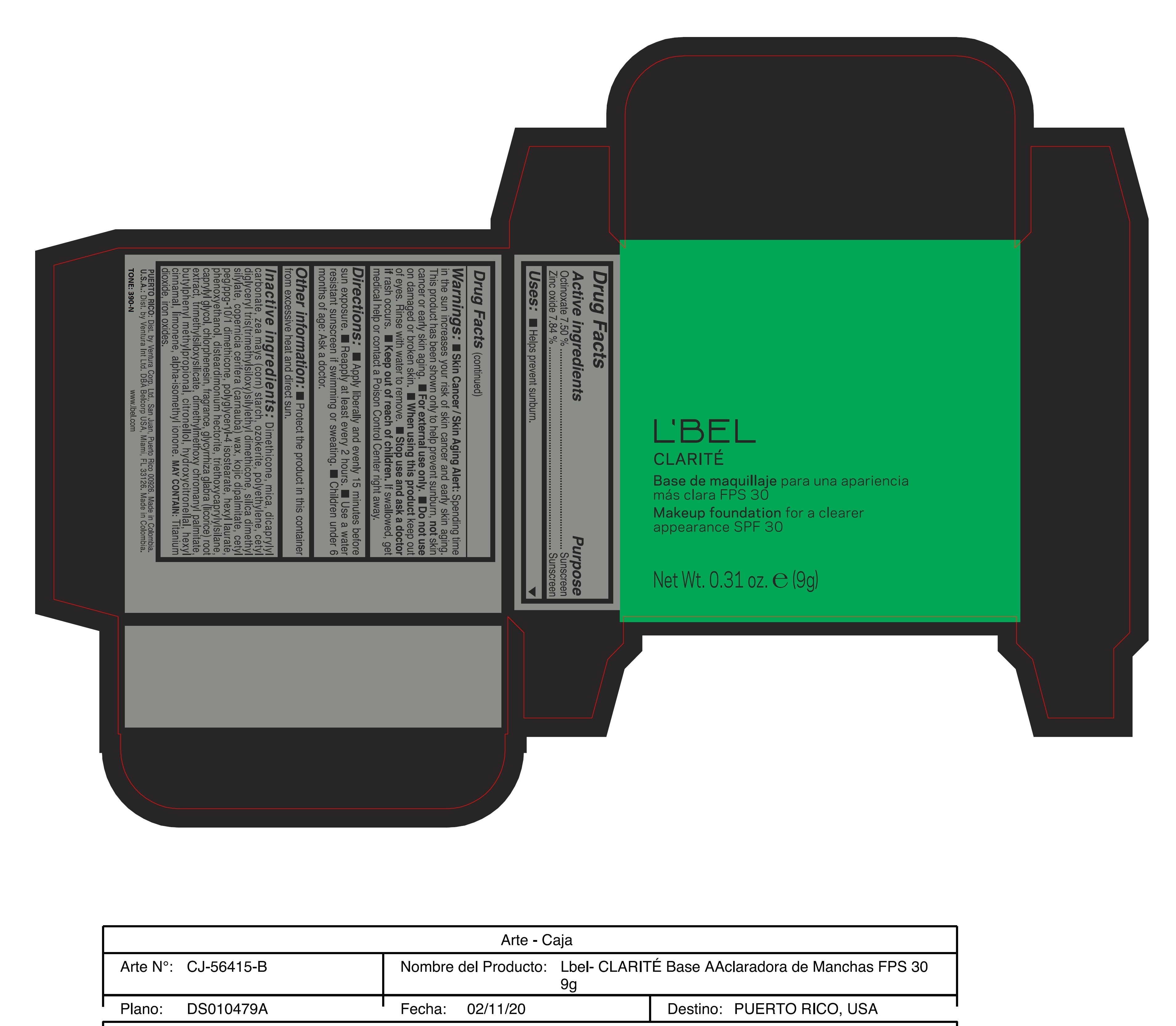
Label: CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 390-N- octinoxate, zinc oxide paste
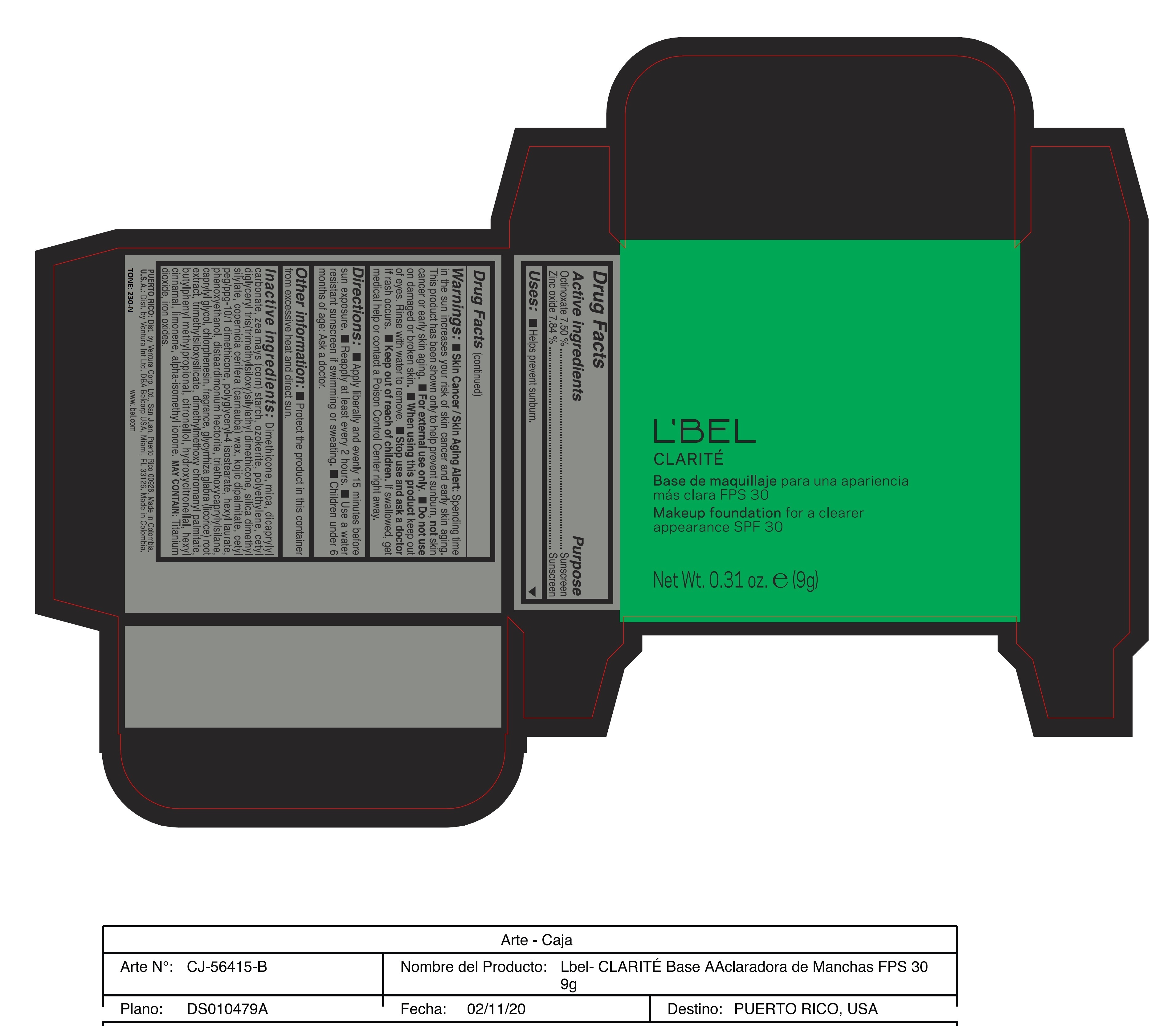
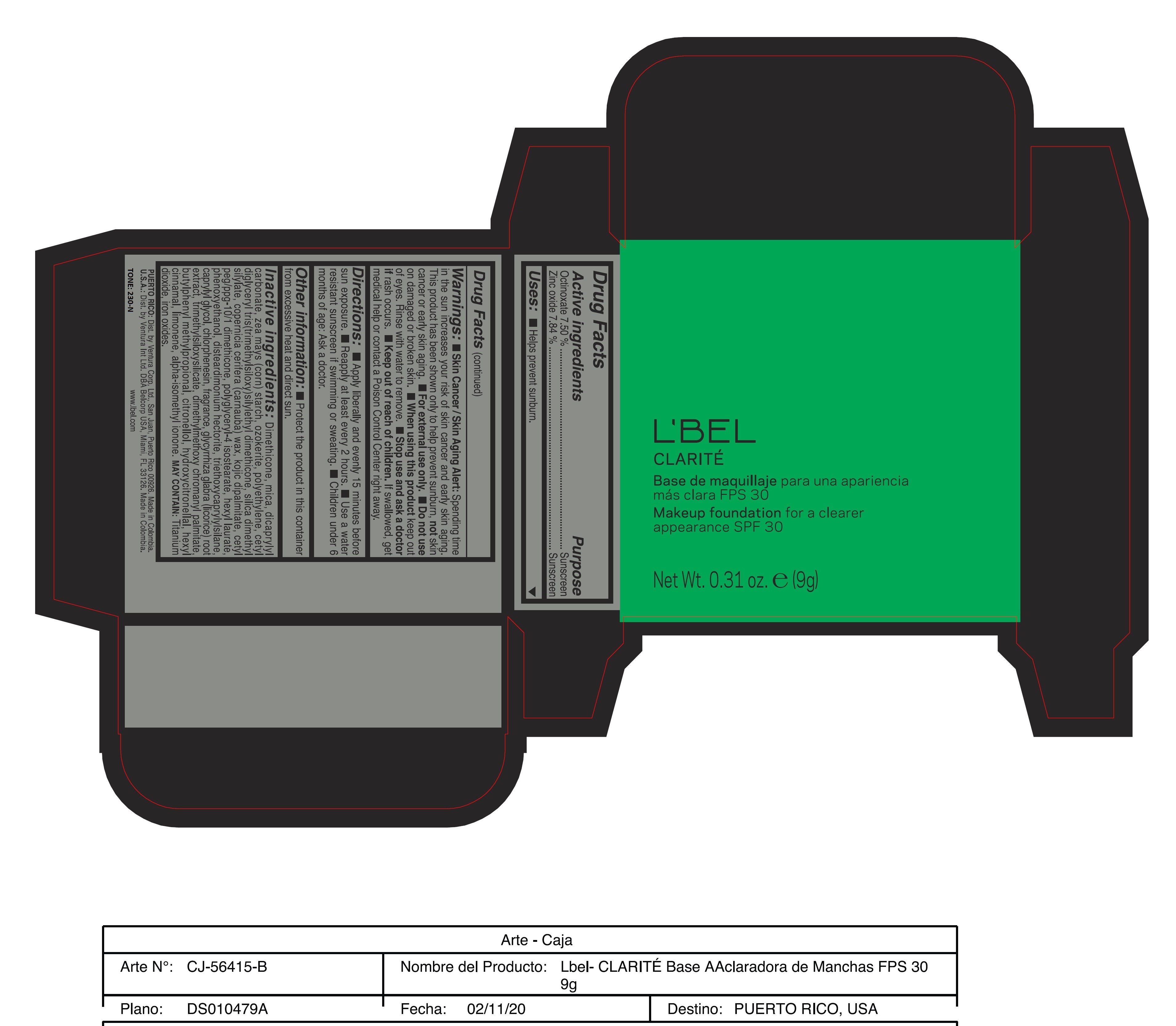
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 230-N- octinoxate, zinc oxide paste
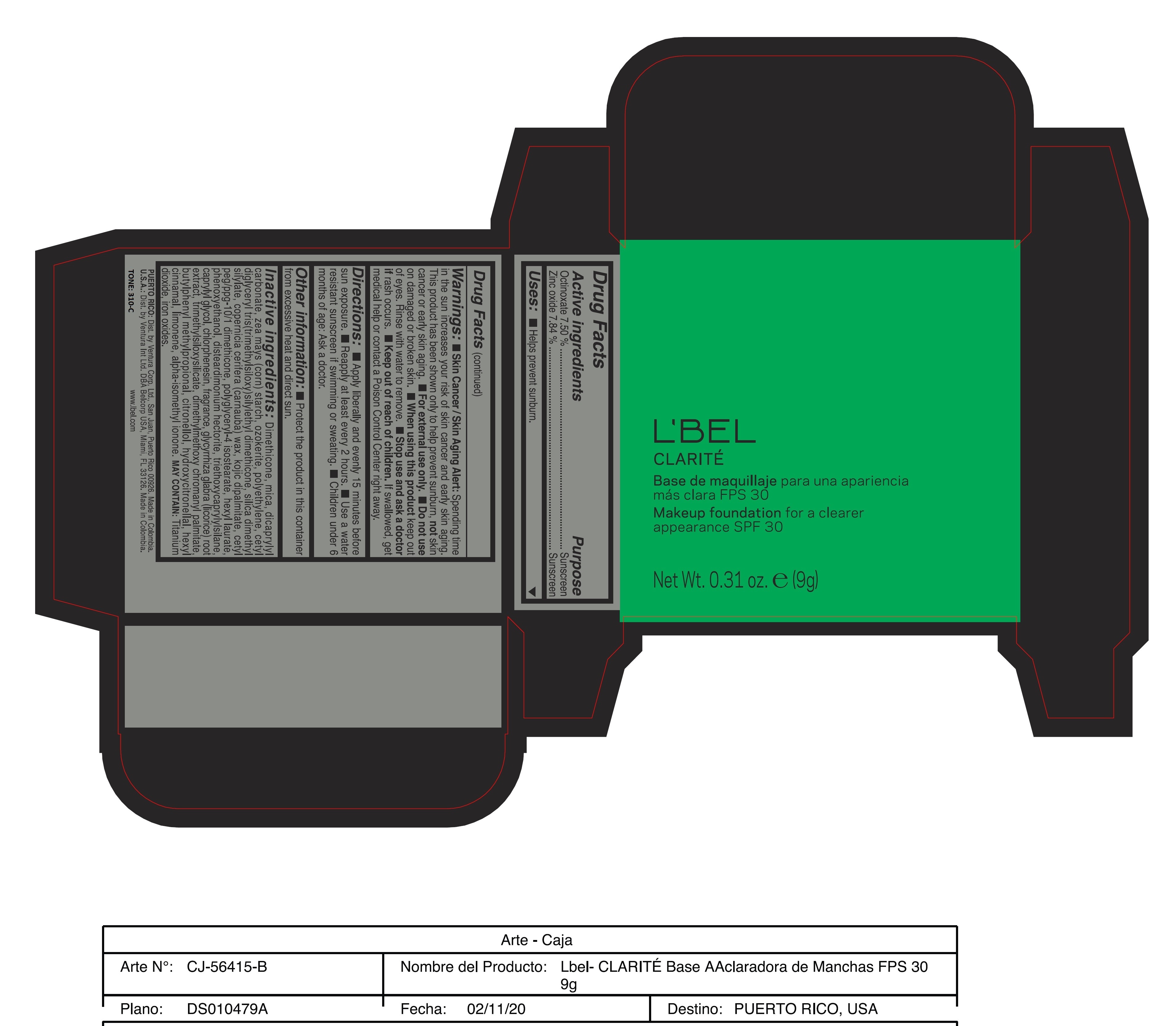
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 310-C- octinoxate, zinc oxide paste
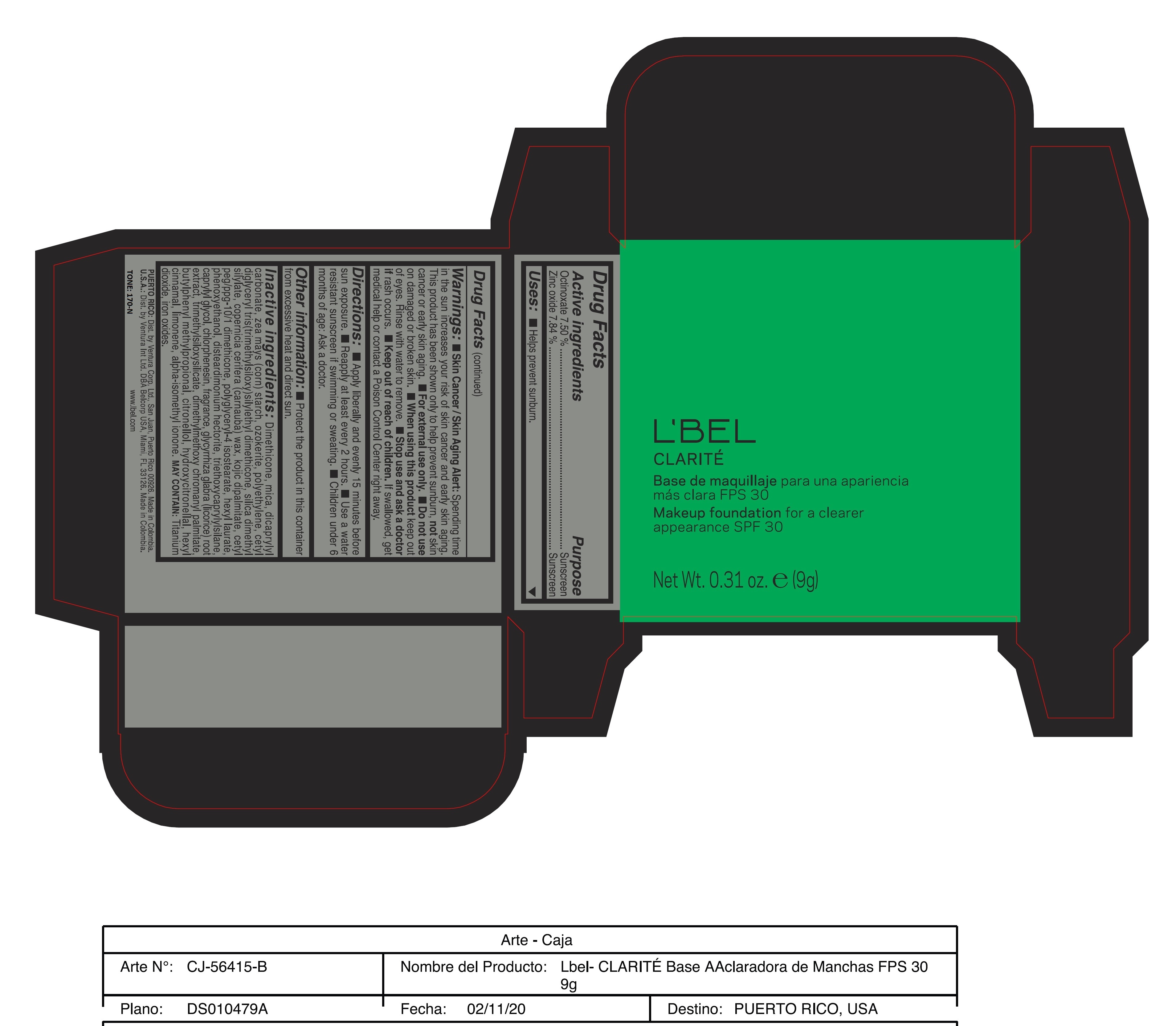
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 170-N- octinoxate, zinc oxide paste
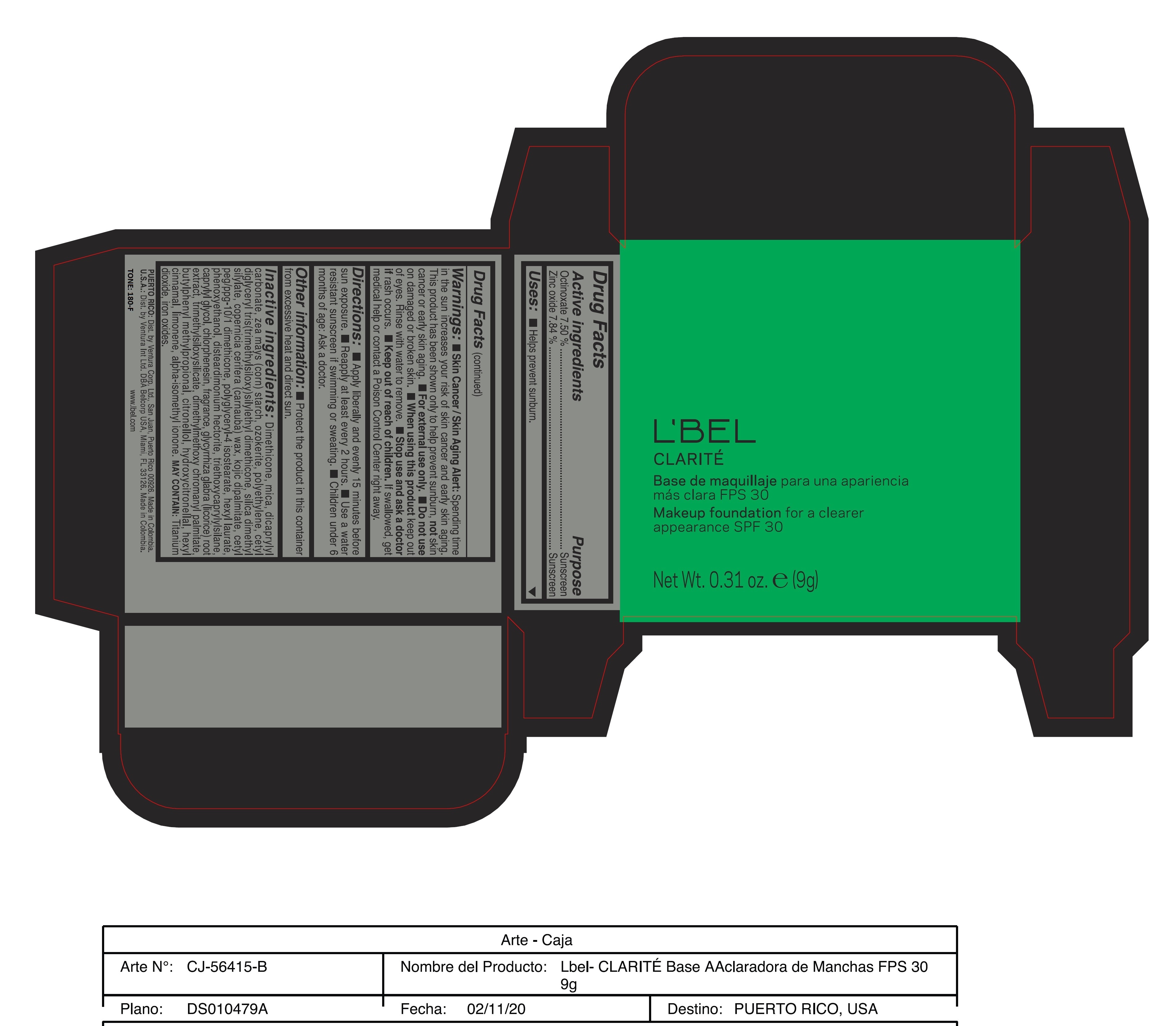
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 180-F- octinoxate, zinc oxide paste
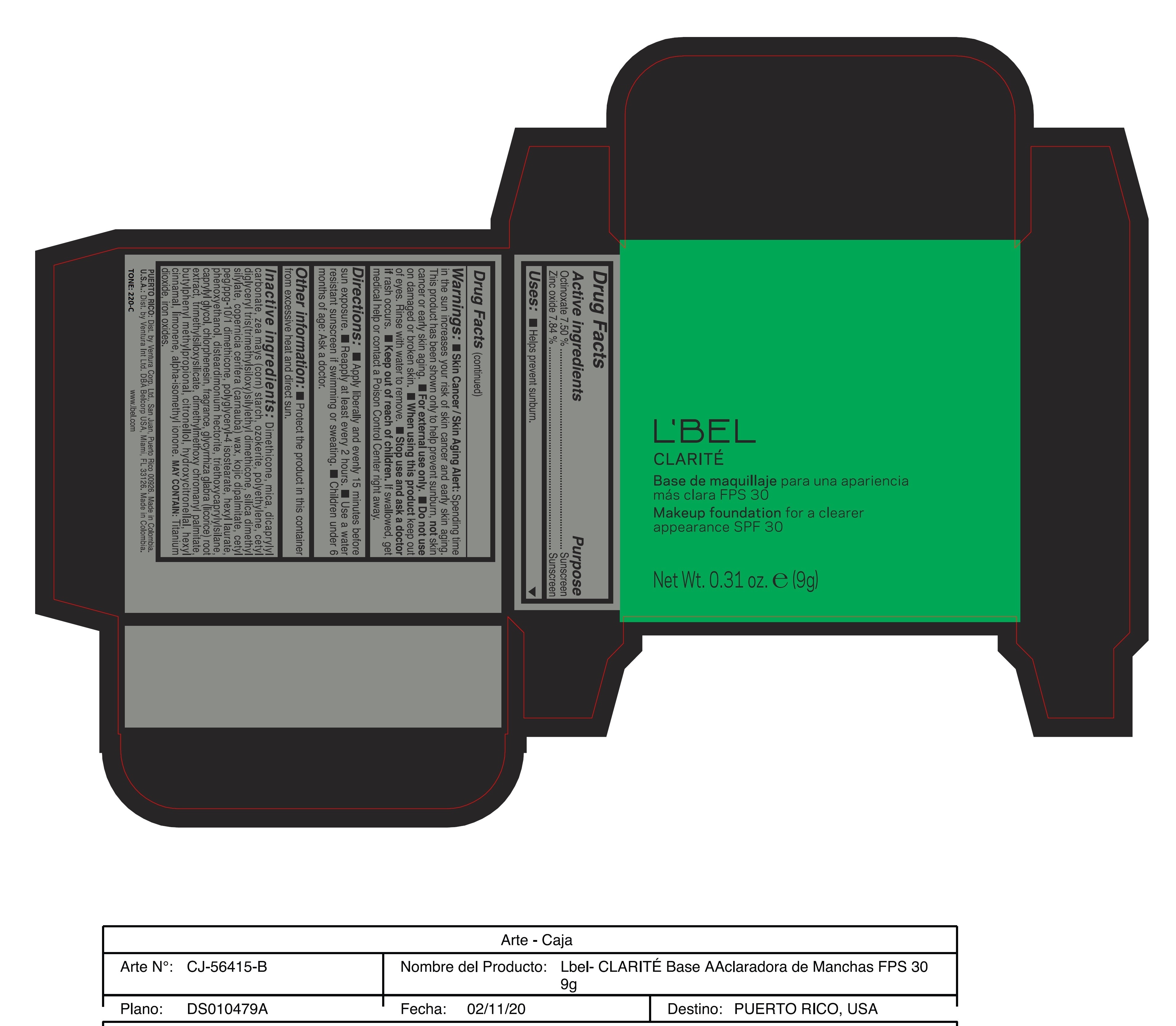
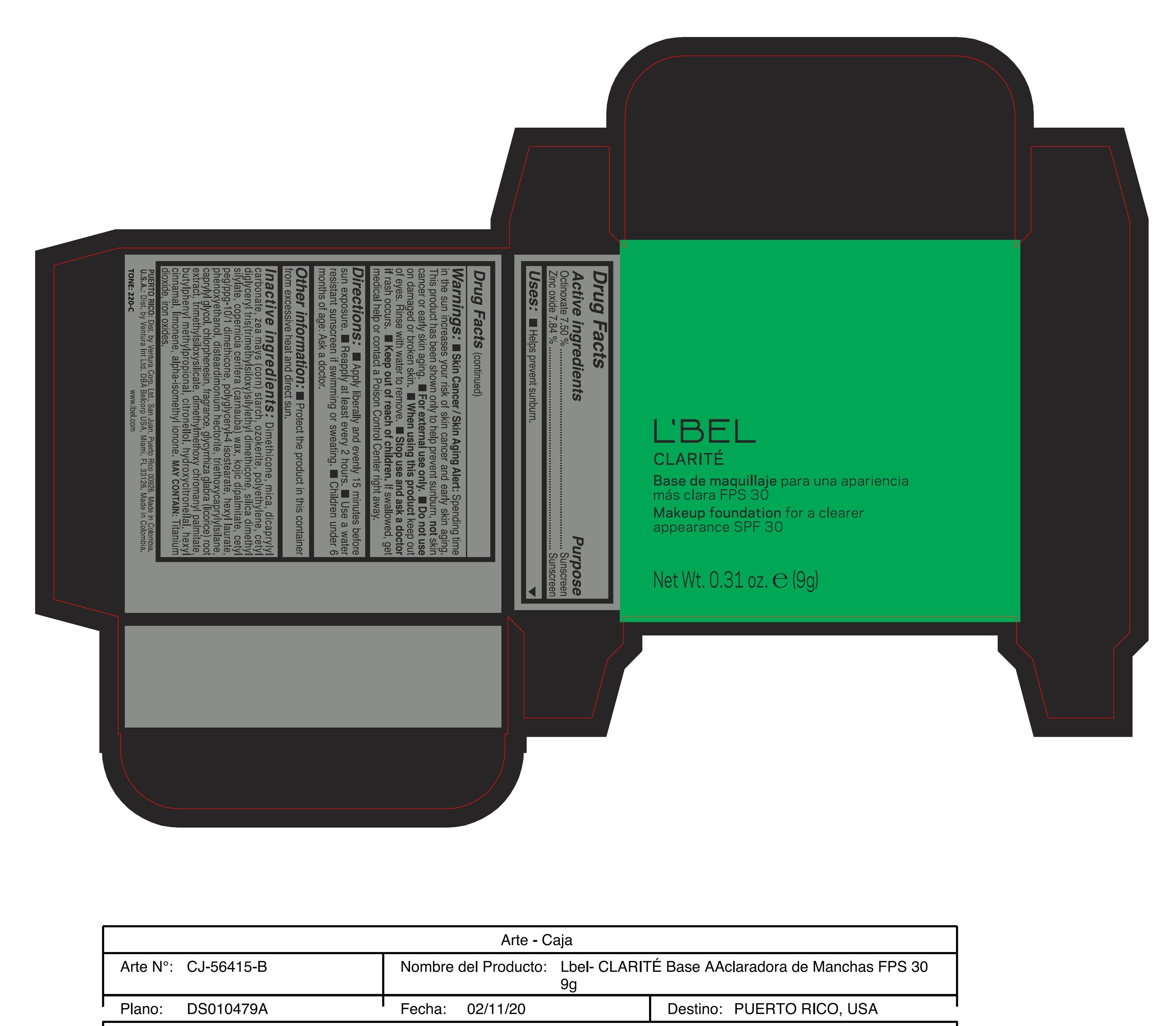
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 220-C- octinoxate, zinc oxide paste
-
NDC Code(s):
14141-207-01,
14141-208-01,
14141-209-01,
14141-210-01, view more14141-211-01, 14141-212-01
- Packager: BEL STAR S A
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove. - DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: Dimethicone, mica, dicaprylyl carbonate, zea mays (corn) starch, ozokerite, polyethylene, cetyl diglyceryl tris(trimethylsiloxy)silylethyl dimethicone, silica dimethyl silylate, copernicia cerifera (carnauba) wax, kojic dipalmitate, cetyl peg/ppg-10/1 dimethicone, polyglyceryl-4 isostearate, hexyl laurate, phenoxyethanol, disteardimonium hectorite, triethoxycaprylylsilane, caprylyl glycol, chlorphenesin, fragrance, glycyrrhiza glabra (licorice) root extract, trimethylsiloxysilicate, dimethylmethoxy chromanyl palmitate, butylphenyl methylpropional, citronellol, hydroxycitronellal, hexyl cinnamal, limonene, alpha-isomethyl ionone.
may contain: titanium dioxide, iron oxides. - 170-N
- 180-F
- 220-C
- 230-N
- 310-C
- 390-N
-
INGREDIENTS AND APPEARANCE
CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 390-N
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-212 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-212-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 230-N
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-210-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 310-C
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-211-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 170-N
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-207 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-207-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 180-F
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-208 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-208-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 CLARITE MAKEUP FOUNDATION FOR A CLEARER APPEARANCE SPF 30 220-C
octinoxate, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-209 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.84 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+/-)- (UNII: 9MC3I34447) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) STARCH, CORN (UNII: O8232NY3SJ) CERESIN (UNII: Q1LS2UJO3A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CARNAUBA WAX (UNII: R12CBM0EIZ) KOJIC DIPALMITATE (UNII: 13N249RWTM) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-209-01 9 g in 1 CASE; Type 0: Not a Combination Product 11/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 11/09/2020 Labeler - BEL STAR S A (880160197) Establishment Name Address ID/FEI Business Operations BEL STAR S A 880160197 manufacture(14141-207, 14141-208, 14141-209, 14141-210, 14141-211, 14141-212)