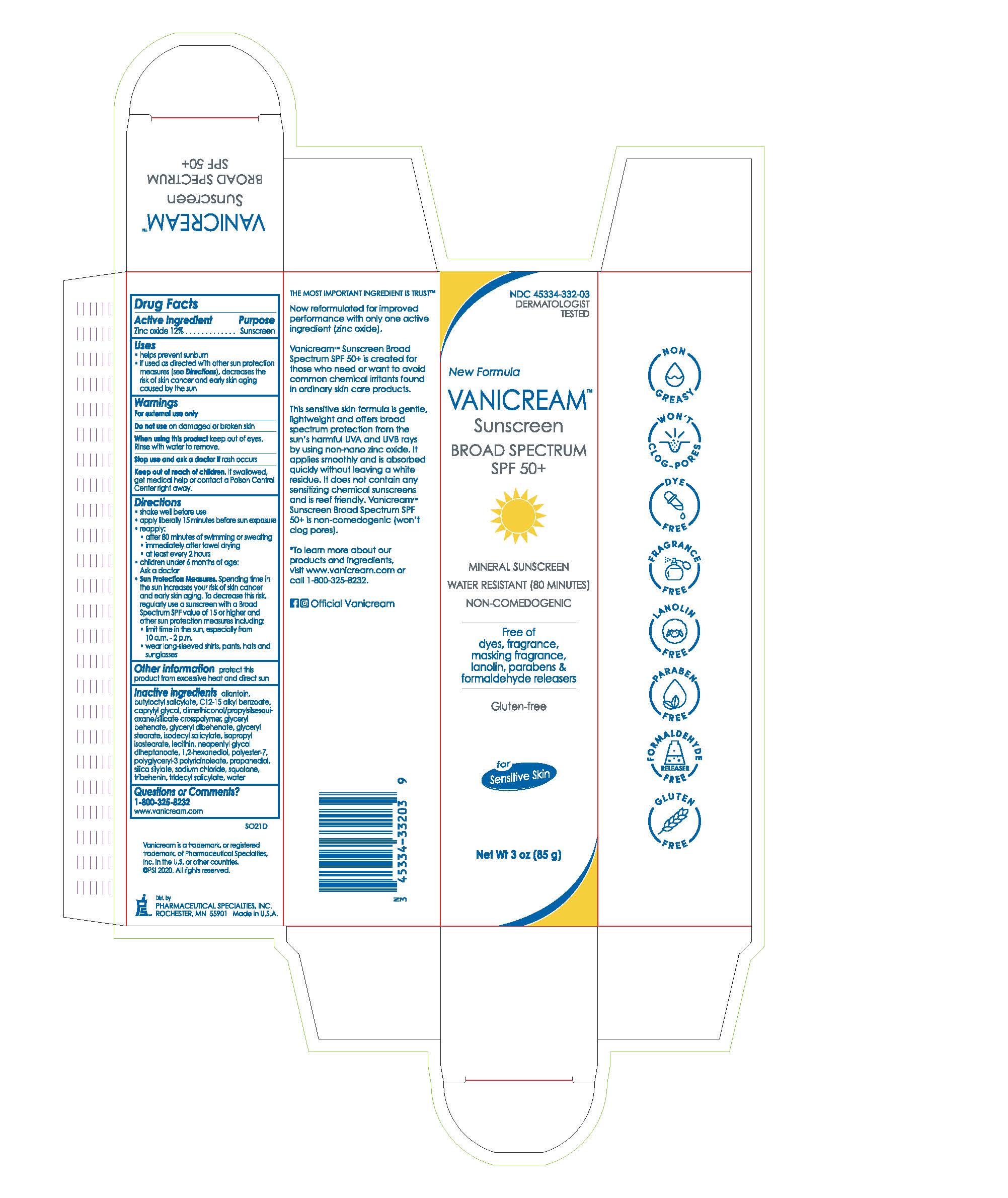
Label: VANICREAM SUNSCREEN BROAD SPECTRUM SPF 50- zinc oxide cream
- NDC Code(s): 45334-332-03
- Packager: Pharmaceutical Specialties, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- shake well before use
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. -2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients allantoin, butyloctyl salicylate, C12-15 alkyl benzoate, caprylyl glycol, dimethiconol/propylsilsesquioxane/silicate crosspolymer, glyceryl behenate, glyceryl dibehenate, glyceryl stearate, isodecyl salicylate, isopropyl isostearate, lecithin, neopentyl glycol diheptanoate, 1,2-hexanediol, polyester-7, polyglyceryl-3 polyricinoleate, propanediol, silica silylate, sodium chloride, squalane, tribehenin, tridecyl salicylate, water
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VANICREAM SUNSCREEN BROAD SPECTRUM SPF 50
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45334-332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 12 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) ALLANTOIN (UNII: 344S277G0Z) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONOL/PROPYLSILSESQUIOXANE/SILICATE CROSSPOLYMER (450000000 MW) (UNII: 9KB5R958PB) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) GLYCERYL 1,3-DIBEHENATE (UNII: 84T2X52XS0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISODECYL SALICYLATE (UNII: S7097PFP4C) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) POLYESTER-7 (UNII: 0841698D2F) SQUALANE (UNII: GW89575KF9) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) PROPANEDIOL (UNII: 5965N8W85T) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) TRIBEHENIN (UNII: 8OC9U7TQZ0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45334-332-03 1 in 1 CARTON 04/05/2021 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/05/2021 Labeler - Pharmaceutical Specialties, Inc. (076499557)