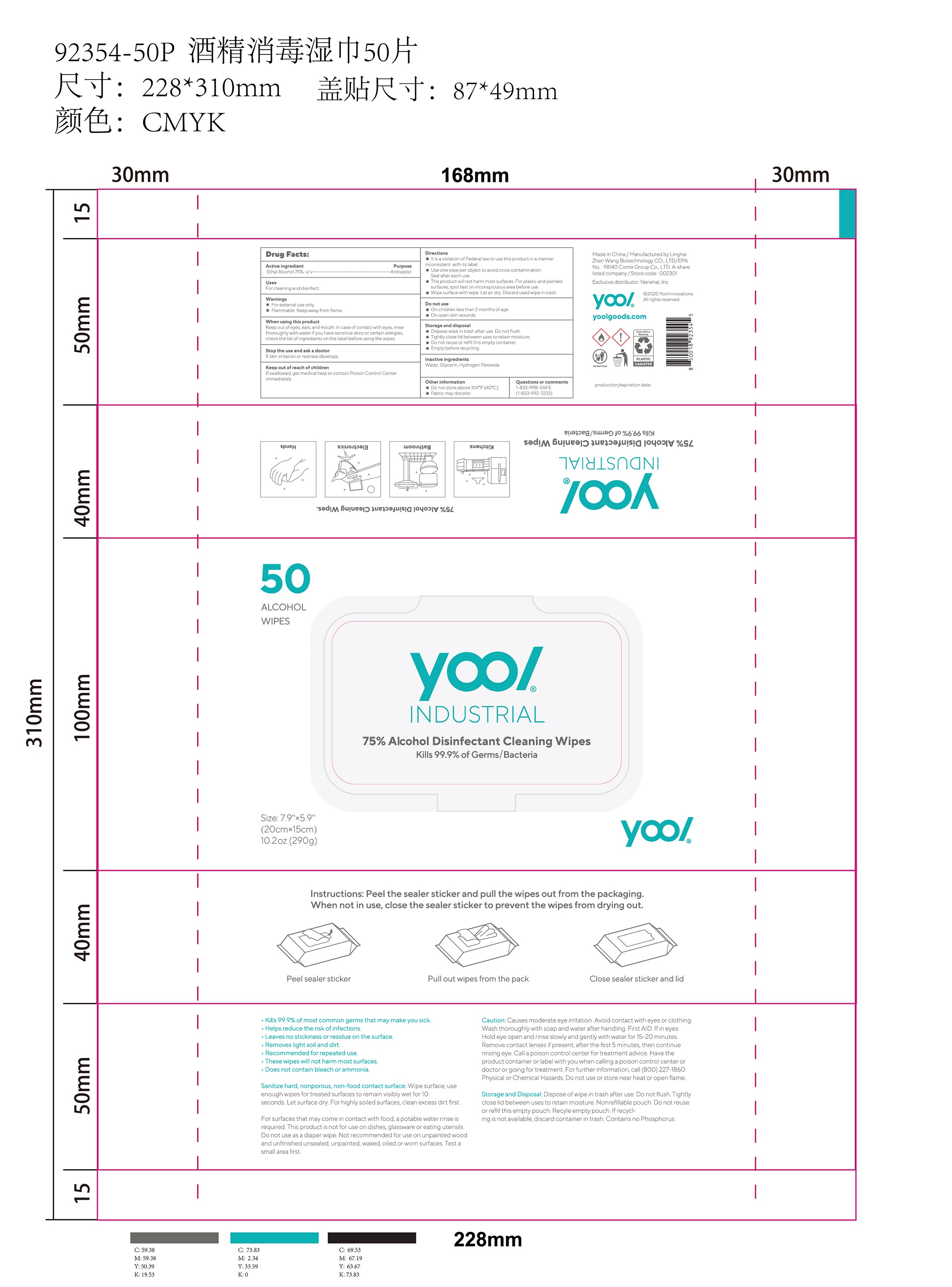
Label: 75% ALCOHOL DISINFECTANT CLEANING WIPES cloth
- NDC Code(s): 75269-006-01
- Packager: LINGHAI ZHANWANG BIOTECHNOLOGY CO.,LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- When using this product
- STOP USE
- Keep out of reach of children
-
Directions
It is a violation of Federal law to use this product in a manner inconsistent with its label.
Use one wipe per object to avoid cross contamination.
Seal after each use.
This product will not harm most surfaces. For plastic and painted surfaces, spot test on inconspicuous area before use.
Wipe surface with wipe. Let air dry. Discard used wipe in trash.
- Do not use
- Storage and disposal
- Inactive ingredients
- Other information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
75% ALCOHOL DISINFECTANT CLEANING WIPES
75% alcohol disinfectant cleaning wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75269-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROGEN PEROXIDE (UNII: BBX060AN9V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75269-006-01 50 in 1 BAG 11/24/2020 1 5.375 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/24/2020 Labeler - LINGHAI ZHANWANG BIOTECHNOLOGY CO.,LTD. (560972943) Registrant - LINGHAI ZHANWANG BIOTECHNOLOGY CO.,LTD. (560972943) Establishment Name Address ID/FEI Business Operations LINGHAI ZHANWANG BIOTECHNOLOGY CO.,LTD. 560972943 manufacture(75269-006)