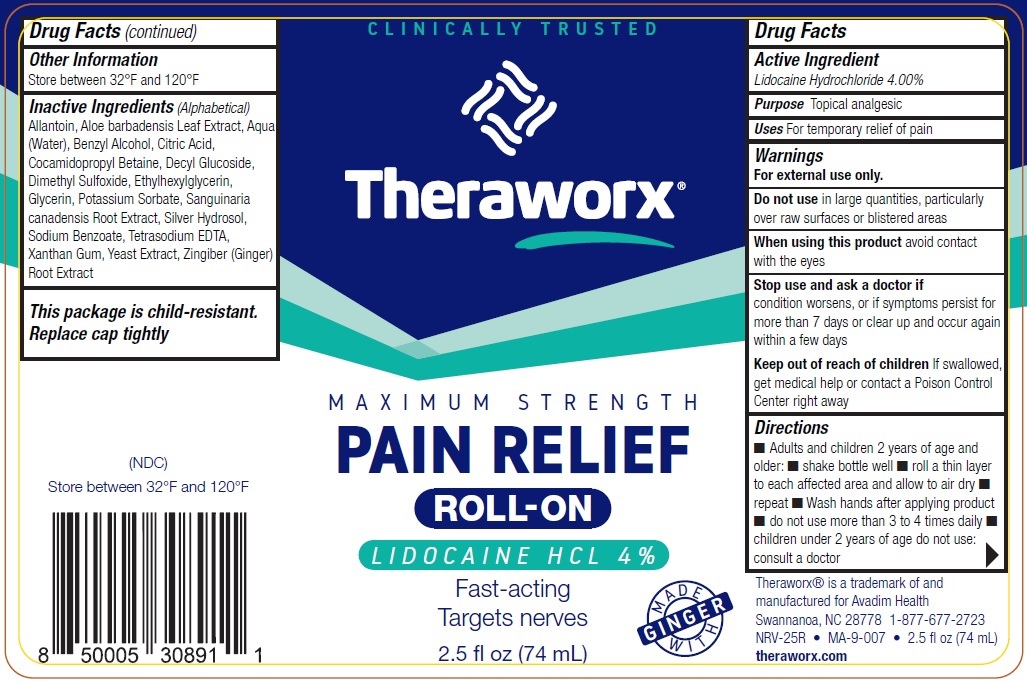
Label: THERAWORX PAIN RELIEF ROLL- ON- LIDOCAINE- lidocaine hydrochloride liquid
- NDC Code(s): 61594-028-00
- Packager: AVADIM HOLDINGS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients (Alphabetical)
Allantoin, Aloe barbadensis Leaf Extract, Aqua (Water), Benzyl Alcohol, Citric Acid, Cocamidopropyl Betaine, Decyl Glucoside, Dimethyl Sulfoxide, Ethylhexylglycerin, Glycerin, Potassium Sorbate, Sanguinaria canadensis Root Extract, Silver Hydrosol, Sodium Benzoate, Tetrasodium EDTA, Xanthan Gum, Yeast Extract, Zingiber (Ginger) Root Extract
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
THERAWORX PAIN RELIEF ROLL- ON- LIDOCAINE
lidocaine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61594-028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) SODIUM BENZOATE (UNII: OJ245FE5EU) EDETATE SODIUM (UNII: MP1J8420LU) XANTHAN GUM (UNII: TTV12P4NEE) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) GINGER (UNII: C5529G5JPQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61594-028-00 1 in 1 CARTON 01/15/2024 1 74 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/15/2024 Labeler - AVADIM HOLDINGS, INC. (118512488)