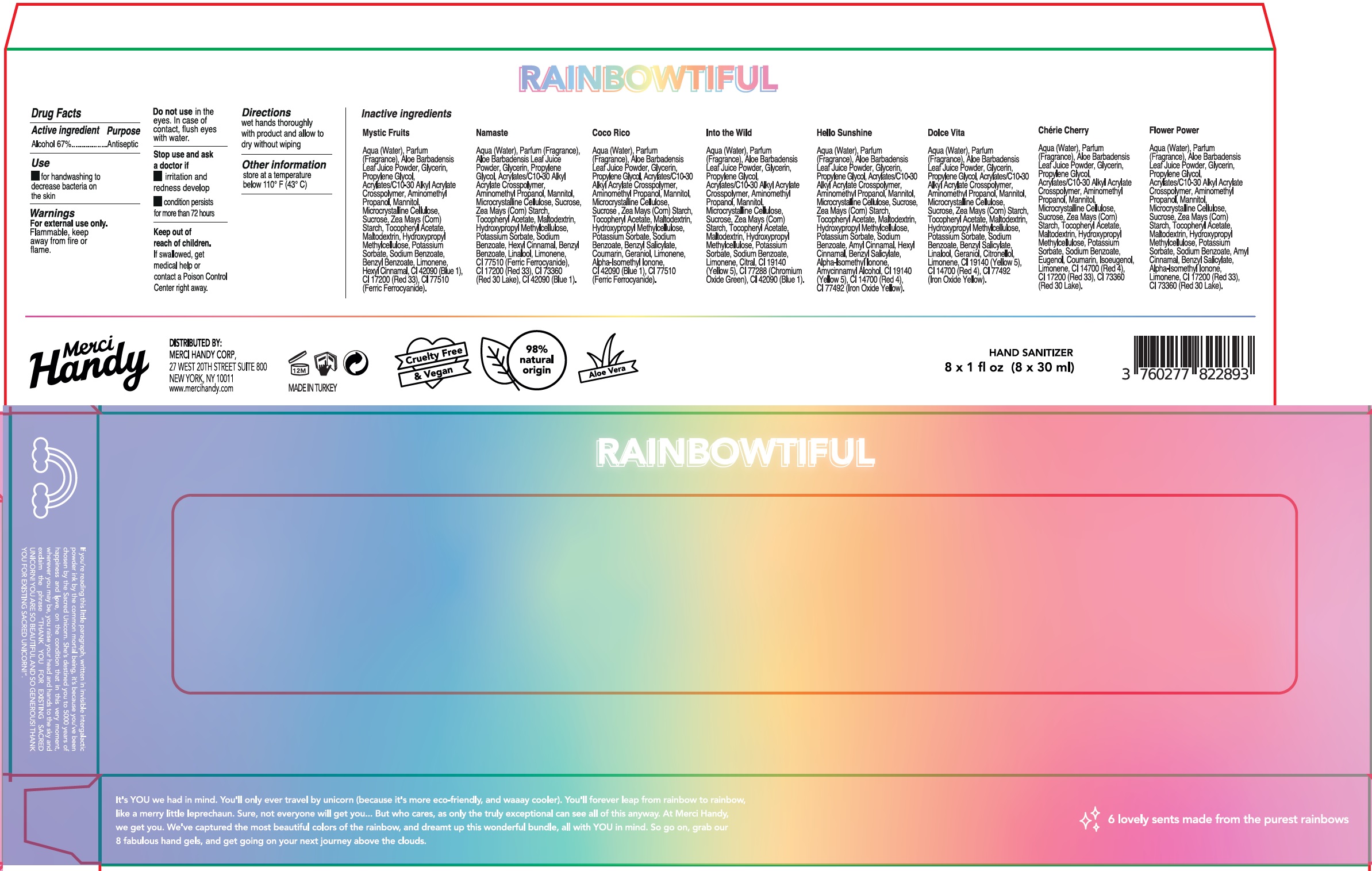
Label: MERCI HANDY RAINBOWTIFUL HAND SANITIZER- alcohol kit
-
NDC Code(s):
72866-000-30,
72866-001-30,
72866-002-30,
72866-005-30, view more72866-006-30, 72866-007-30, 72866-008-30, 72866-010-30, 72866-013-01
- Packager: MERCI HANDY CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
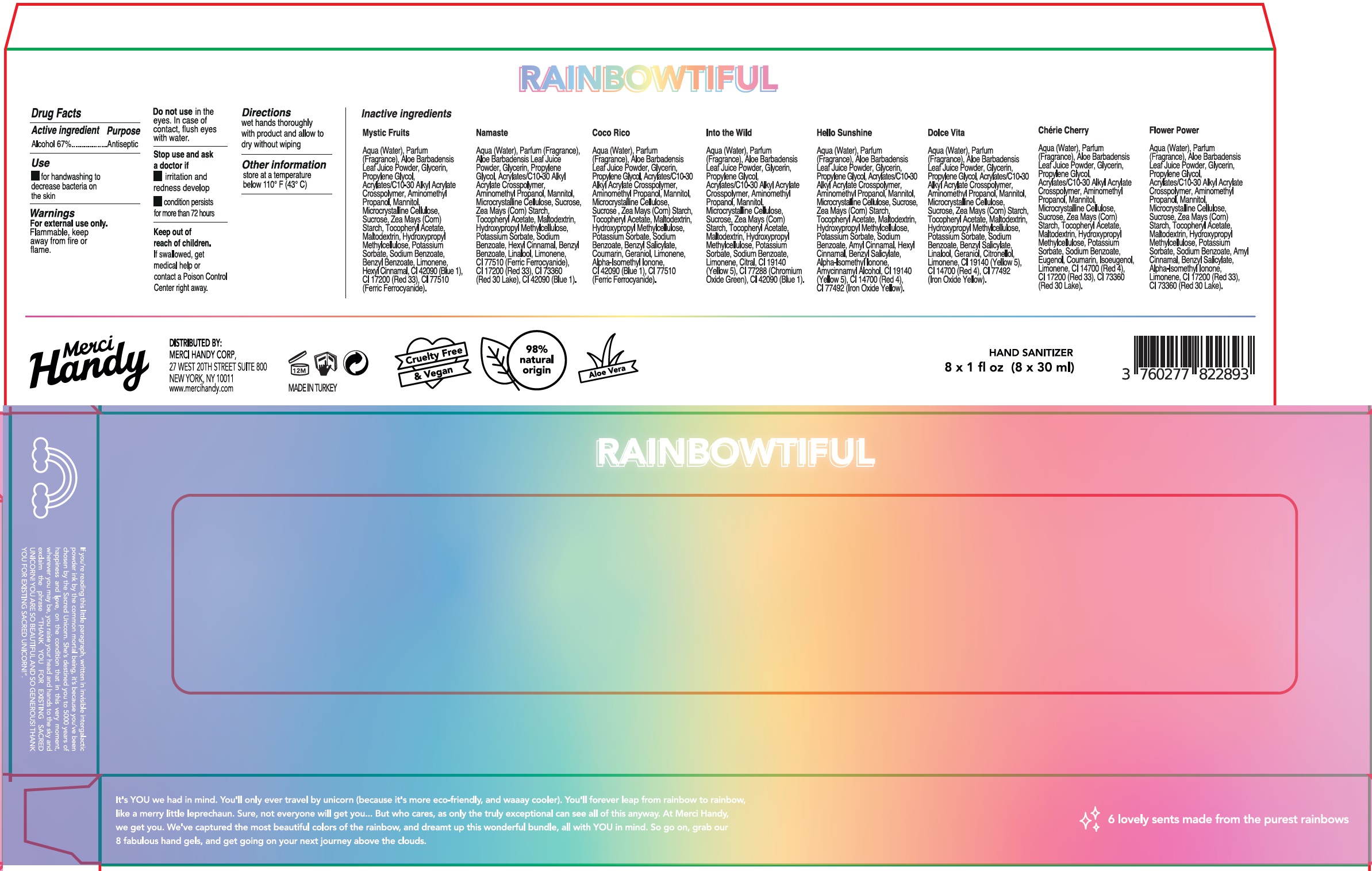
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Limonene, Hexyl Cinnamal, FD&C Blue No.1, D&C Red No. 33, Ferric Ferrocyanide.
- QUESTIONS OR COMMENTS?
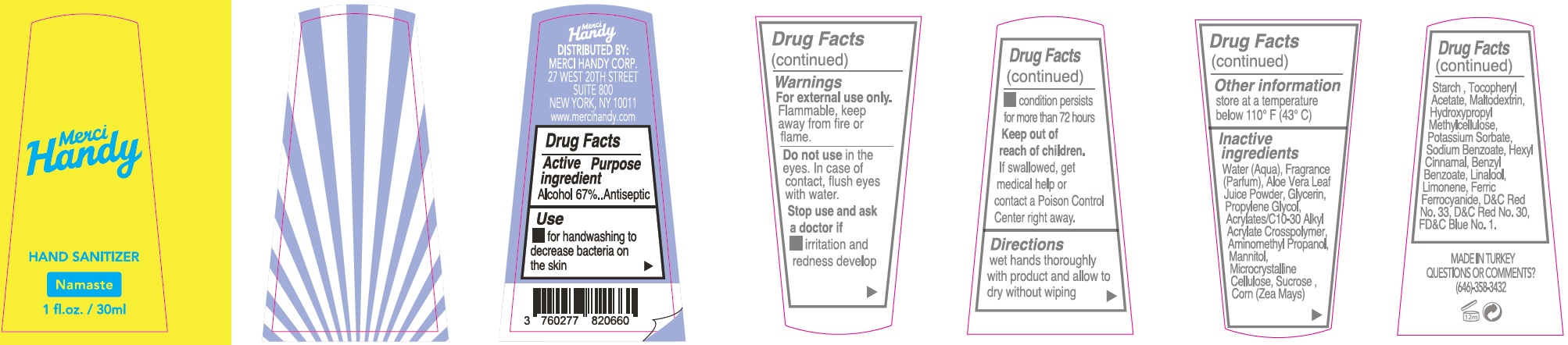
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Hexyl Cinnamal, Benzyl Salicylate, Linalool, Limonene, Ferric Ferrocyanide, D&C Red No. 33, D&C Red No. 30, FD&C Blue No.1.
- QUESTIONS OR COMMENTS?
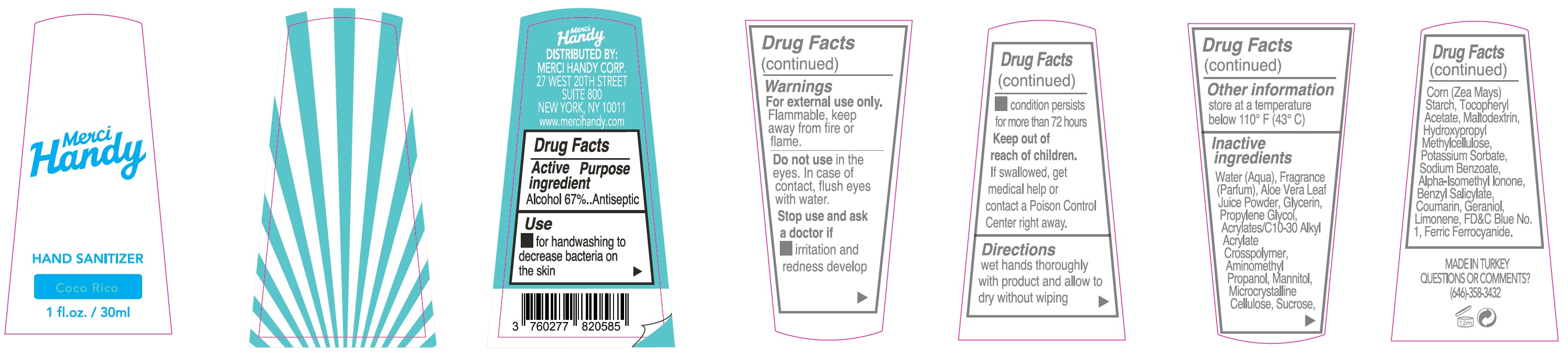
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Alpha-Isomethyl Ionone, Benzyl Salicylate, Coumarin, Geraniol, Limonene, FD&C Blue No. 1, Ferric Ferrocyanide.
- QUESTIONS OR COMMENTS?
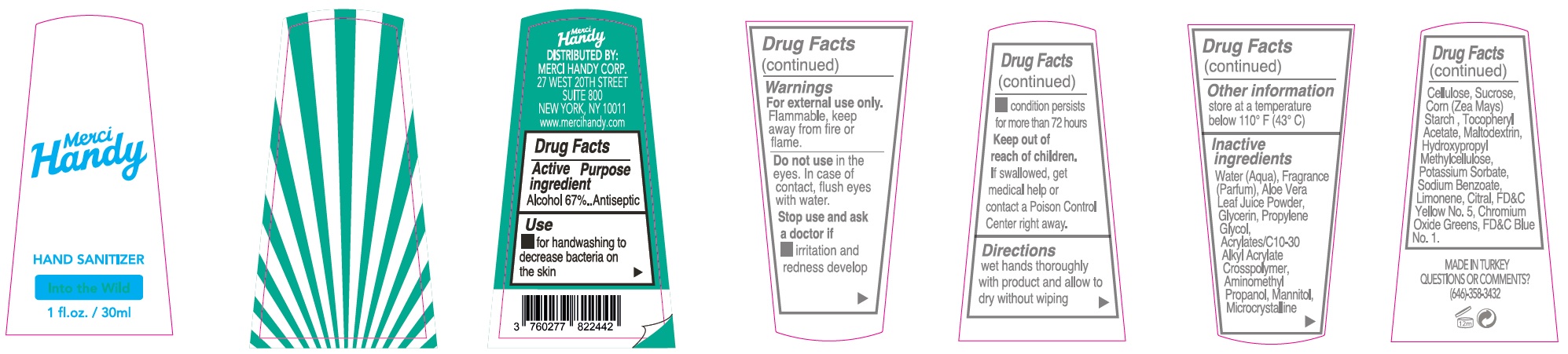
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Limonene, Citral, FD&C Yellow No. 5, Chromium Oxide Greens, FD&C Blue No. 1
- QUESTIONS OR COMMENTS?
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Amyl Cinnamal, Hexyl Cinnamal, Benzyl Salicylate, Alpha-Isomethyl Ionone, Amycinnamyl Alcohol, FD&C Yellow No.5, FD&C Red No. 4, Iron Oxides.
- QUESTIONS OR COMMENTS?
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Benzyl Salicylate, Linalool, Geraniol, Citronellol, Limonene, FD&C Yellow No. 5, FD&C Red No. 4, Iron Oxides.
- QUESTIONS OR COMMENTS?
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Eugenol, Coumarin, Isoeugenol, Limonene, FD&C Red No. 4, D&C Red No. 33, D&C Red No. 30
- QUESTIONS OR COMMENTS?
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Fragrance (Parfum), Aloe Vera Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Corn (Zea Mays) Starch, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Amyl Cinnamal, Benzyl Salicylate, Alpha-Isomethyl Ionone, Limonene, D&C Red No. 33, D&C Red No. 30.
- QUESTIONS OR COMMENTS?
- Package Labeling:Kit
- Package Labeling: MYSTIC FRUITS
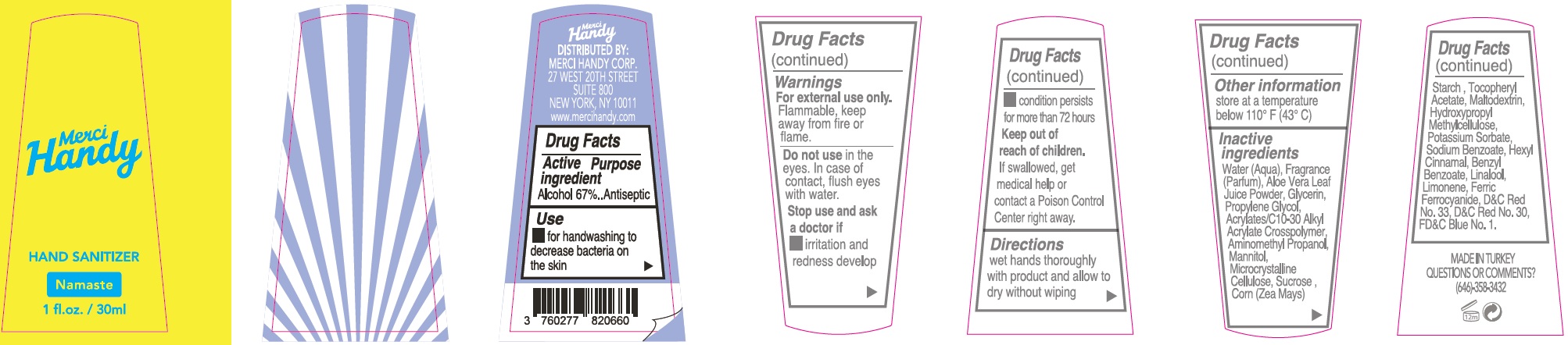
- Package Labeling: NAMASTE
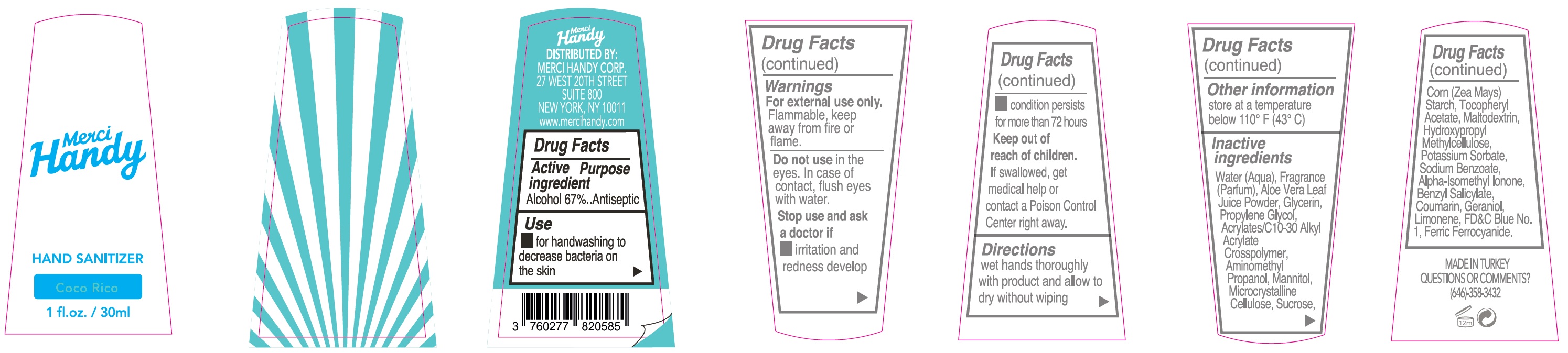
- Package Labeling: COCO RICO
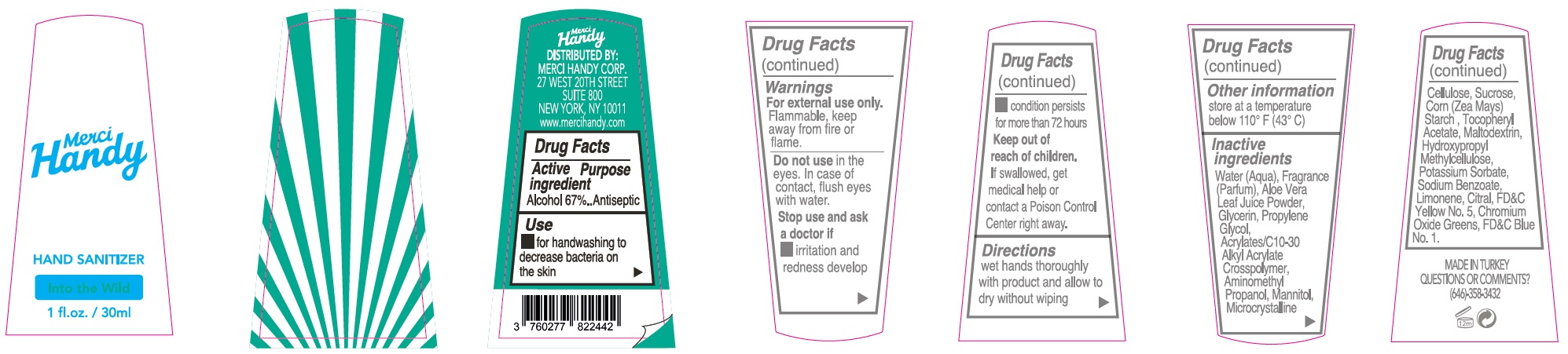
- Package Labeling:INTO THE WILD
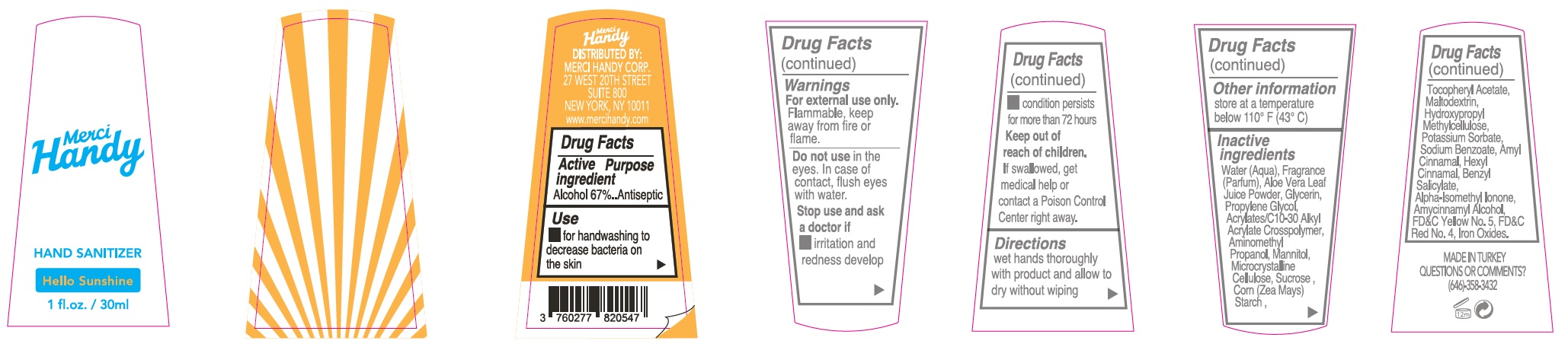
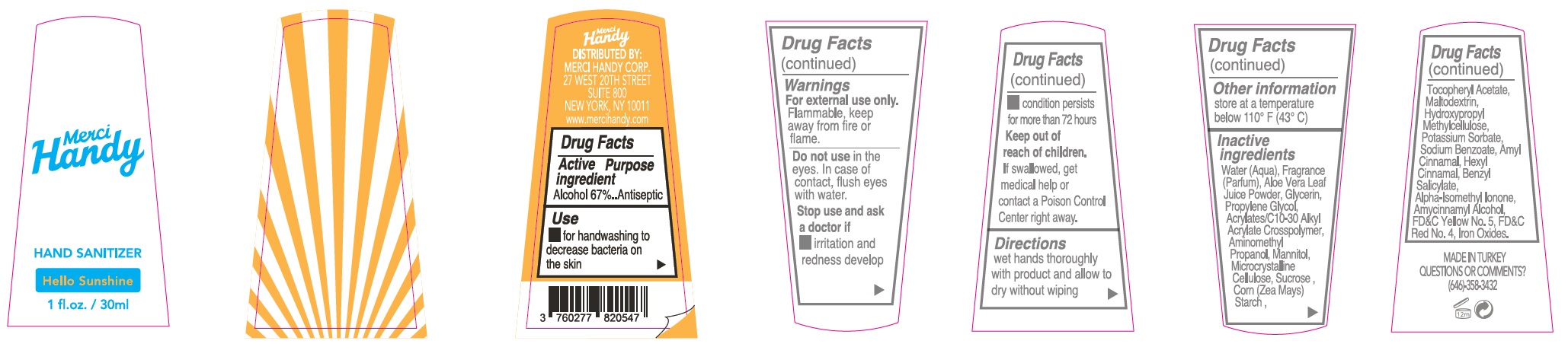
- Package Labeling:HELLO SUNSHINE
- Package Labeling:DOLCE VITA
- Package Labeling:Cherie Cherry
- Package Labeling:FLOWER POWER
-
INGREDIENTS AND APPEARANCE
MERCI HANDY RAINBOWTIFUL HAND SANITIZER
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72866-013 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-013-01 1 in 1 KIT 01/01/2021 06/30/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 1 BOTTLE 30 mL Part 3 1 BOTTLE 30 mL Part 4 1 BOTTLE 30 mL Part 5 1 BOTTLE 30 mL Part 6 1 BOTTLE 30 mL Part 7 1 BOTTLE 30 mL Part 8 1 BOTTLE 30 mL Part 1 of 8 HAND SANITIZER, MYSTIC FRUITS
alcohol gelProduct Information Item Code (Source) NDC:72866-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) LIMONENE, (+)- (UNII: GFD7C86Q1W) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FERRIC FERROCYANIDE (UNII: TLE294X33A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-005-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 2 of 8 HAND SANITIZER, NAMASTE
alcohol gelProduct Information Item Code (Source) NDC:72866-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC FERROCYANIDE (UNII: TLE294X33A) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-002-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 3 of 8 HAND SANITIZER, COCO RICO
alcohol gelProduct Information Item Code (Source) NDC:72866-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL SALICYLATE (UNII: WAO5MNK9TU) COUMARIN (UNII: A4VZ22K1WT) GERANIOL (UNII: L837108USY) LIMONENE, (+)- (UNII: GFD7C86Q1W) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC FERROCYANIDE (UNII: TLE294X33A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-006-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 4 of 8 HAND SANITIZER, INTO THE WILD
alcohol gelProduct Information Item Code (Source) NDC:72866-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) LIMONENE, (+)- (UNII: GFD7C86Q1W) CITRAL (UNII: T7EU0O9VPP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) CHROMIC OXIDE (UNII: X5Z09SU859) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-008-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 5 of 8 HAND SANITIZER, HELLO SUNSHINE
alcohol gelProduct Information Item Code (Source) NDC:72866-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) BENZYL SALICYLATE (UNII: WAO5MNK9TU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) .ALPHA.-AMYLCINNAMYL ALCOHOL (UNII: DKB52S61GU) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-000-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 6 of 8 HAND SANITIZER, DOLCE VITA
alcohol gelProduct Information Item Code (Source) NDC:72866-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LINALOOL, (+/-)- (UNII: D81QY6I88E) GERANIOL (UNII: L837108USY) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+)- (UNII: GFD7C86Q1W) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-007-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 7 of 8 HAND SANITIZER CHERIE CHERRY
alcohol gelProduct Information Item Code (Source) NDC:72866-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.67 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) EUGENOL (UNII: 3T8H1794QW) COUMARIN (UNII: A4VZ22K1WT) ISOEUGENOL (UNII: 5M0MWY797U) LIMONENE, (+)- (UNII: GFD7C86Q1W) FD&C RED NO. 4 (UNII: X3W0AM1JLX) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-010-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Part 8 of 8 HAND SANITIZER, FLOWER POWER
alcohol gelProduct Information Item Code (Source) NDC:72866-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 670 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) BENZYL SALICYLATE (UNII: WAO5MNK9TU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C RED NO. 30 (UNII: 2S42T2808B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-001-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2021 06/30/2025 Labeler - MERCI HANDY CORPORATION (118006306)