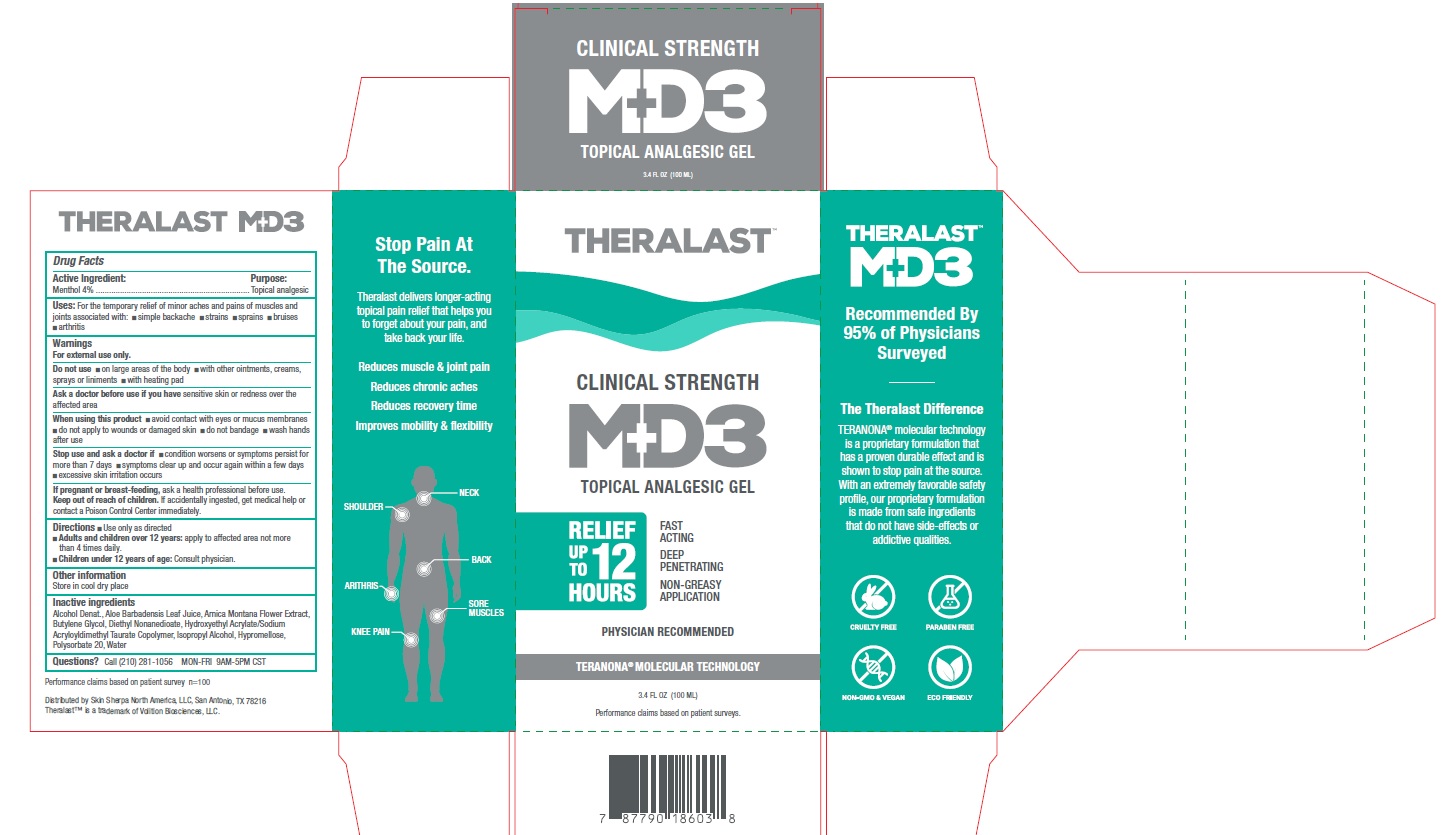
Label: THERALAST CLINICAL STRENGTH MD3 TOPICAL ANALGESIC- menthol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 80967-002-01 - Packager: SKIN SHERPA NORTH AMERICA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 25, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Do not use
- on large areas of the body
- with other ointments, creams, sprays or liniments
- with heating pad
When using this product
- avoid contact with eyes or mucus membranes
- do not apply to wounds or damaged skin
- do not bandage
- wash hands after use
- Directions
- Other information
- Inactive ingredients
- Questions?
- Company Information
- Product Packaging
-
INGREDIENTS AND APPEARANCE
THERALAST CLINICAL STRENGTH MD3 TOPICAL ANALGESIC
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80967-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) DIETHYL AZELATE (UNII: 4E9QQ39A4X) ISOPROPYL ALCOHOL (UNII: ND2M416302) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80967-002-01 1 in 1 BOX 11/15/2020 1 100 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/15/2020 Labeler - SKIN SHERPA NORTH AMERICA LLC (117719003)