Label: QUIP ANTICAVITY FLUORIDE CONCENTRATE- sodium fluoride mouthwash
-
Contains inactivated NDC Code(s)
NDC Code(s): 69261-005-03, 69261-005-04, 69261-005-05 - Packager: Quip NYC Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
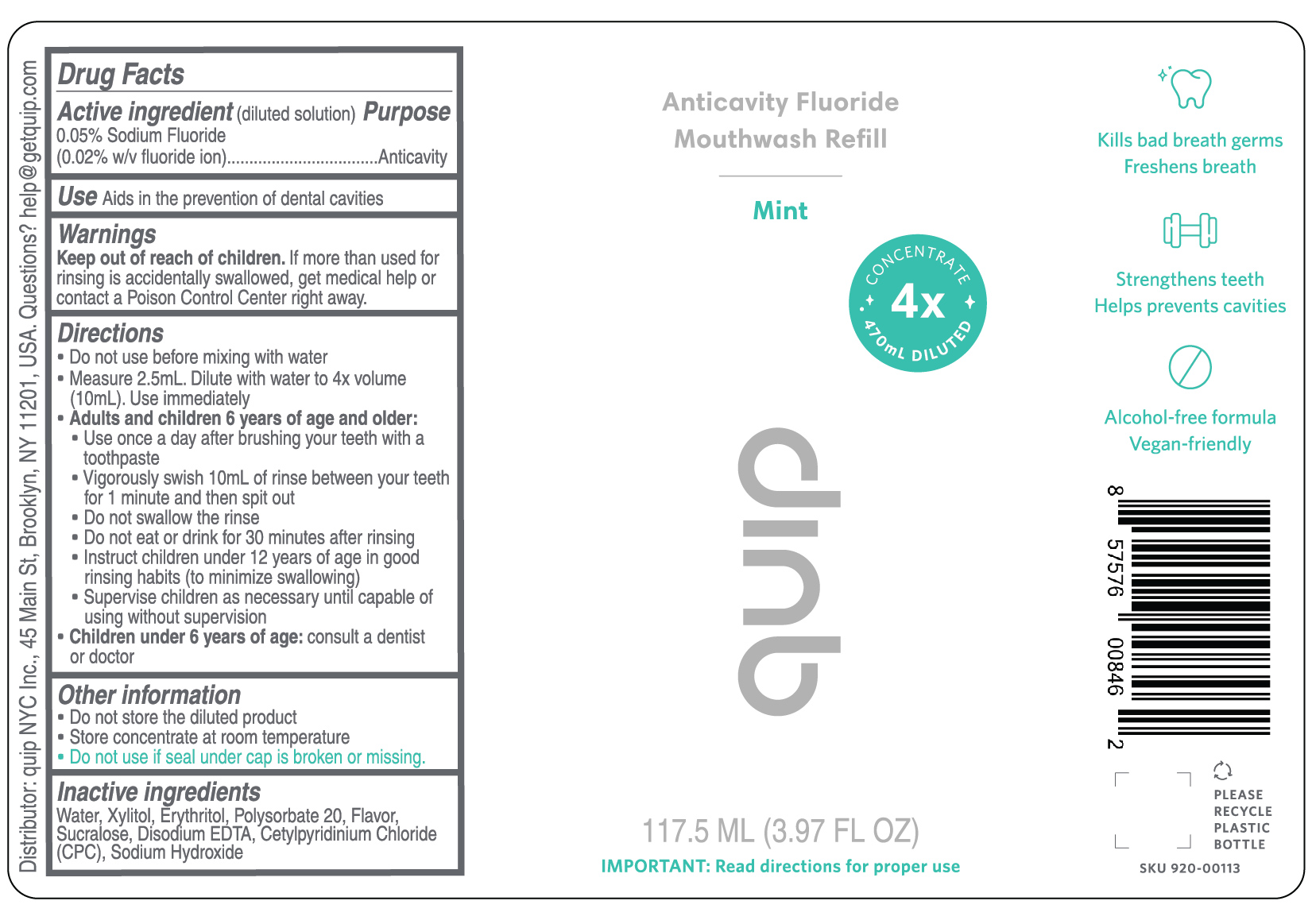
Directions
- Do not use before mixing with water
- Measure 2.5ml. Dilute with water to 4x volume (10ml). Use immediately
- Adults and children 6 years of age and older:
- Use once a day after brushing your teeth with a toothpaste
- Vigorously swish 10ml of rinse between your teeth for 1 minute and then spit out
- Do not swallow the rinse
- Do not eat or drink for 30 minutes after rinsing
- Supervise children as necessary until capable of using without supervision
- Children under 6 years of age: consult a dentist or doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- BOTTLE
- RETAIL CARTON
-
STARTER KIT
QUIP
Mouthwash Starter Kit
Refillable Dispenser & Cup
One pump for perfect daily dose
Mint Anticavity Fluoride Mouthwash Refill
Kills bad breath germs
Freshens breath
Strengthens teeth
Helps prevent cavities
Alcohol-free formula
Vegan-friendly4X CONCENTRATE 470mL DILUTED
117.5 ML (3.97 FL OZ)
IMPORTANT: Read directions for proper use
-
INGREDIENTS AND APPEARANCE
QUIP ANTICAVITY FLUORIDE CONCENTRATE
sodium fluoride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69261-005 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ERYTHRITOL (UNII: RA96B954X6) WATER (UNII: 059QF0KO0R) POLYSORBATE 20 (UNII: 7T1F30V5YH) XYLITOL (UNII: VCQ006KQ1E) SODIUM HYDROXIDE (UNII: 55X04QC32I) SUCRALOSE (UNII: 96K6UQ3ZD4) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) Product Characteristics Color yellow (Clear to Yellow Clear) Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69261-005-04 1 in 1 CARTON 01/25/2021 1 117.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69261-005-03 117.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2021 3 NDC:69261-005-05 1 in 1 PACKAGE 01/25/2021 3 117.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 01/25/2021 Labeler - Quip NYC Inc. (079453380)