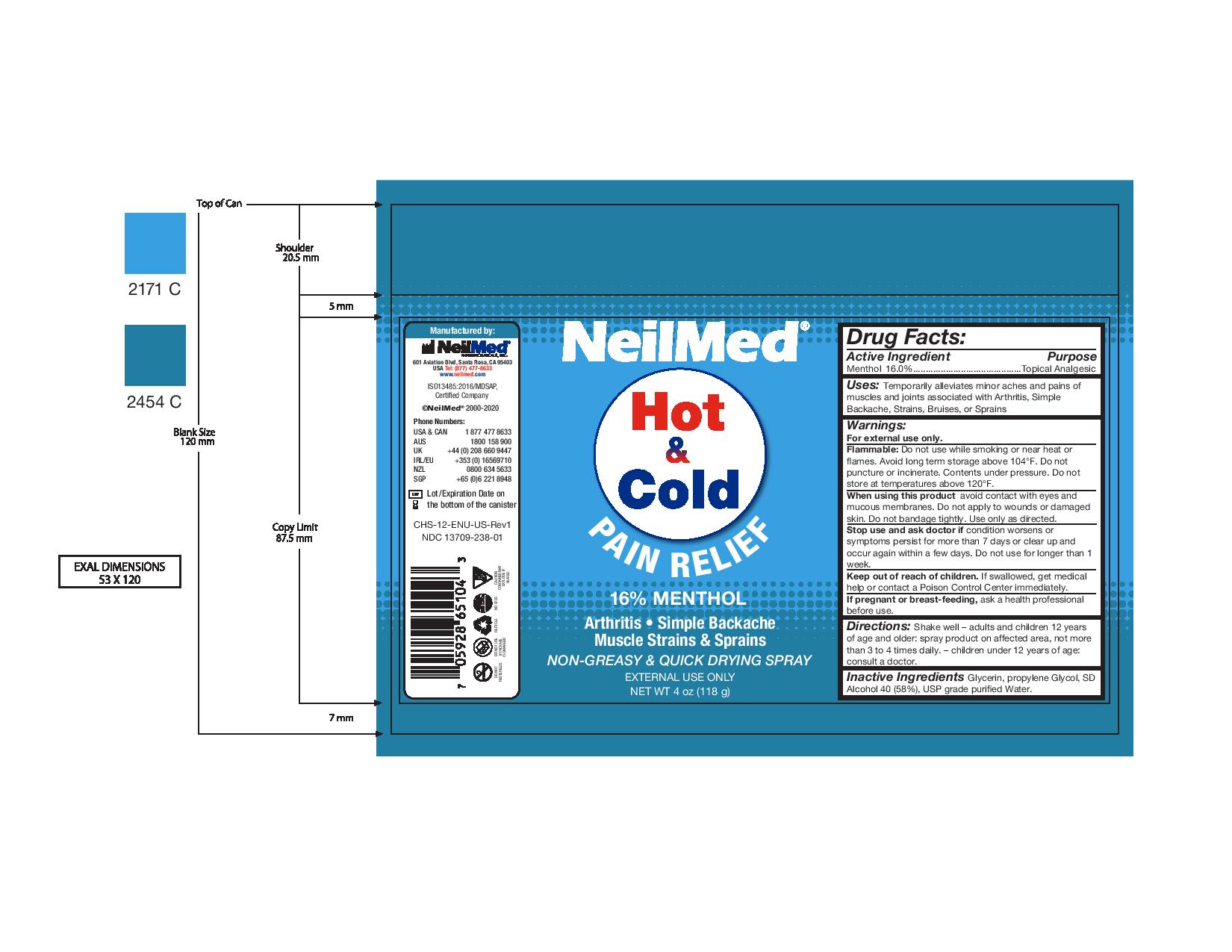
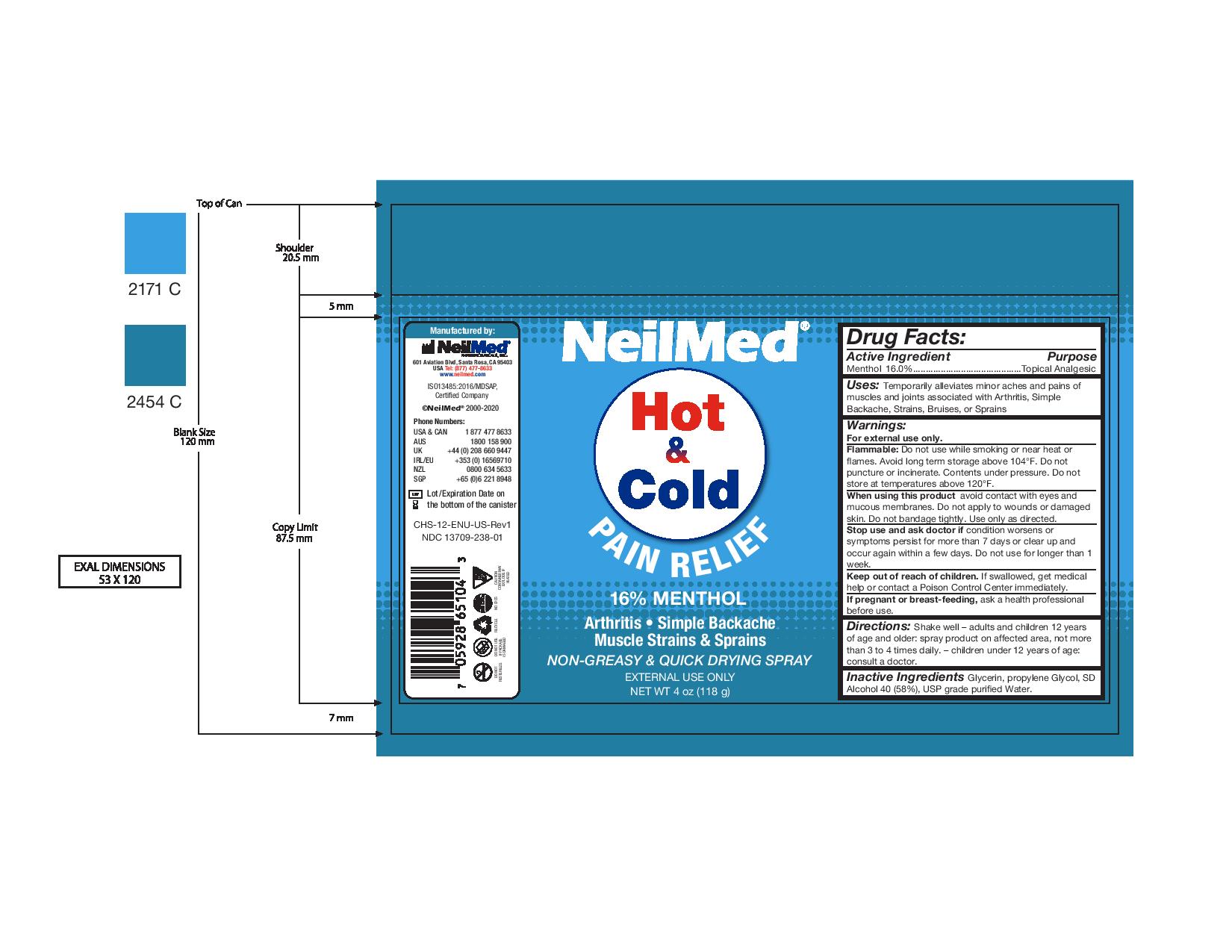
Label: NEILMED HOT AND COLD PAIN RELIEF- pain relief spray spray
- NDC Code(s): 13709-238-01
- Packager: NeilMed Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Directions
-
Warnings
For external use only.
Flammable: Do not use while smoking or near heat or flames. Avoid long term storage above 104°F. Do not puncture or incinerate. Contents under pressure. Do not store at temperatures above 120°F.
When using this product avoid contact with eyes and mucous membranes. Do not apply to wounds or damaged skin. Do not bandage tightly. Use only as directed.
Stop use and ask doctor if condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days. Do not use for longer than 1 week.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
If pregnant or breast-feeding, ask a health professional before use. - Inactive Ingredients
- Uses
- Warnings: Keep out of reach of children.
- Drug Facts:
- Drug Facts:
- Package Label - NeilMed Hot & Cold Pain Relief
-
INGREDIENTS AND APPEARANCE
NEILMED HOT AND COLD PAIN RELIEF
pain relief spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-238 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.16 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-238-01 118 g in 1 CAN; Type 0: Not a Combination Product 11/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/11/2020 Labeler - NeilMed Pharmaceuticals Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals, Inc 799295915 manufacture(13709-238)