Label: EIGHT HOUR LIP SPF 15- petrolatum, padimate o, oxybenzone lipstick
- NDC Code(s): 10967-679-61
- Packager: REVLON
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
-
Inactive Ingredients
RICINUS COMMUNIS (CASTOR) SEED OIL, OZOKERITE, GLYCERYL ROSINATE, CETYL LACTATE, LANOLIN OIL, MYRISTYL LACTATE, CETYL ACETATE, EUPHORBIA CERIFERA (CANDELILLA) WAX/CANDELILLA CERA/CIRE DE CANDELILLA, COPERNICIA CERIFERA (CARNAUBA) WAX/CERA CARNAUBA/CIRE DE CARNAUBA, MYRISTYL ALCOHOL, ACETYLATED LANOLIN ALCOHOL, BEESWAX/CERA ALBA/CIRE D'ABEILLE, CAPRYLYL GLYCOL, GLYCINE SOJA (SOYBEAN) OIL, METHYLSTYRENE/VINYLTOLUENE COPOLYMER, MICROCRYSTALLINE WAX/CERA MICROCRISTALLINA/CIRE MICROCRISTALLINE, PARFUM/FRAGRANCE, TOCOPHEROL, ZEA MAYS (CORN) OIL, BHT, CITRAL, CITRONELLOL, GERANIOL, LIMONENE, LINALOOL, PHENOXYETHANOL, IRON OXIDES (CI 77491, CI 77492, CI 77499), TITANIUM DIOXIDE (CI 77891).
- Uses
-
Warnings
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.- For external use only
- Do not use on damaged or broken skin
- Stop use and ask a doctor if rash occurs or if condition lasts more than 7 days.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- Directions for Use
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
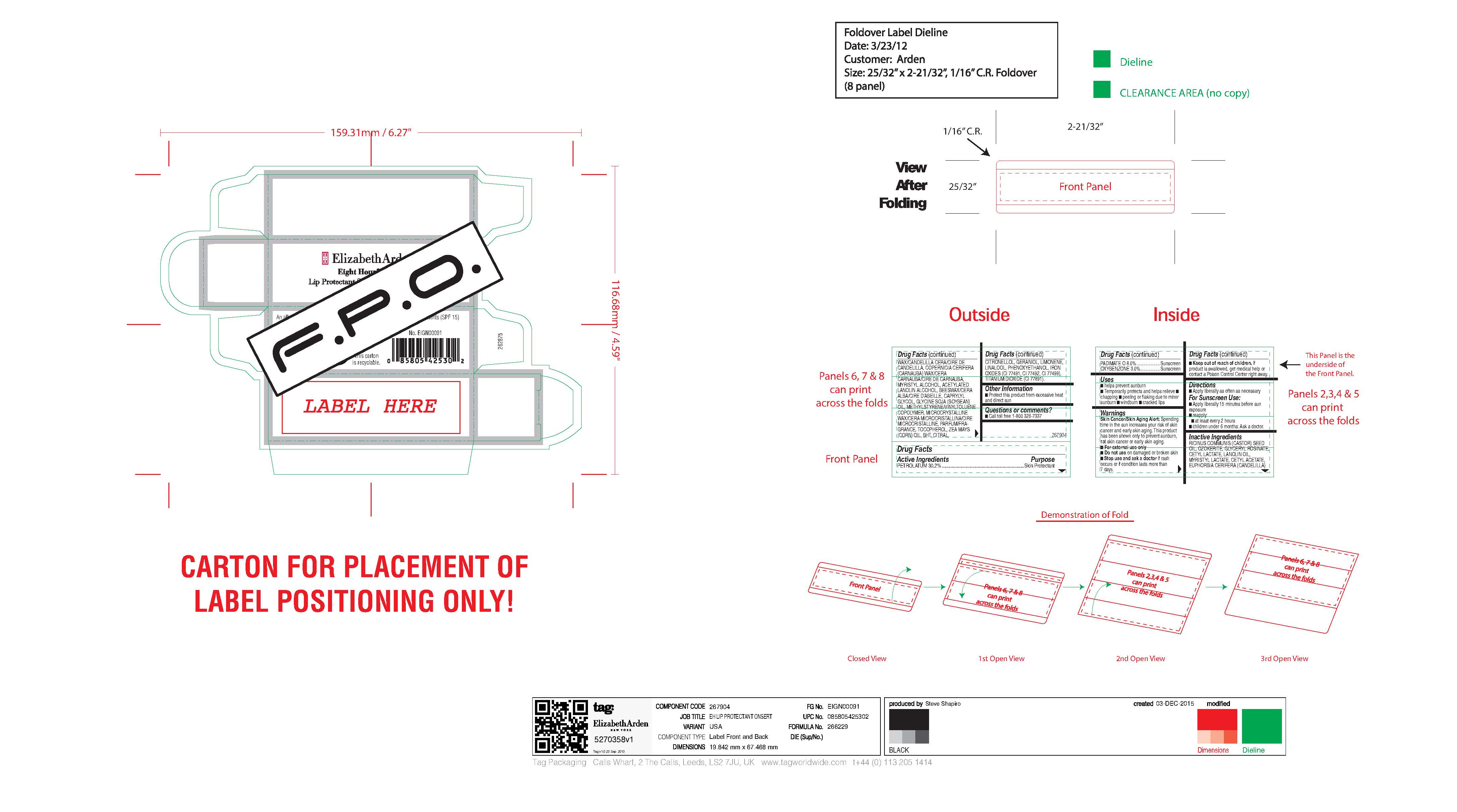
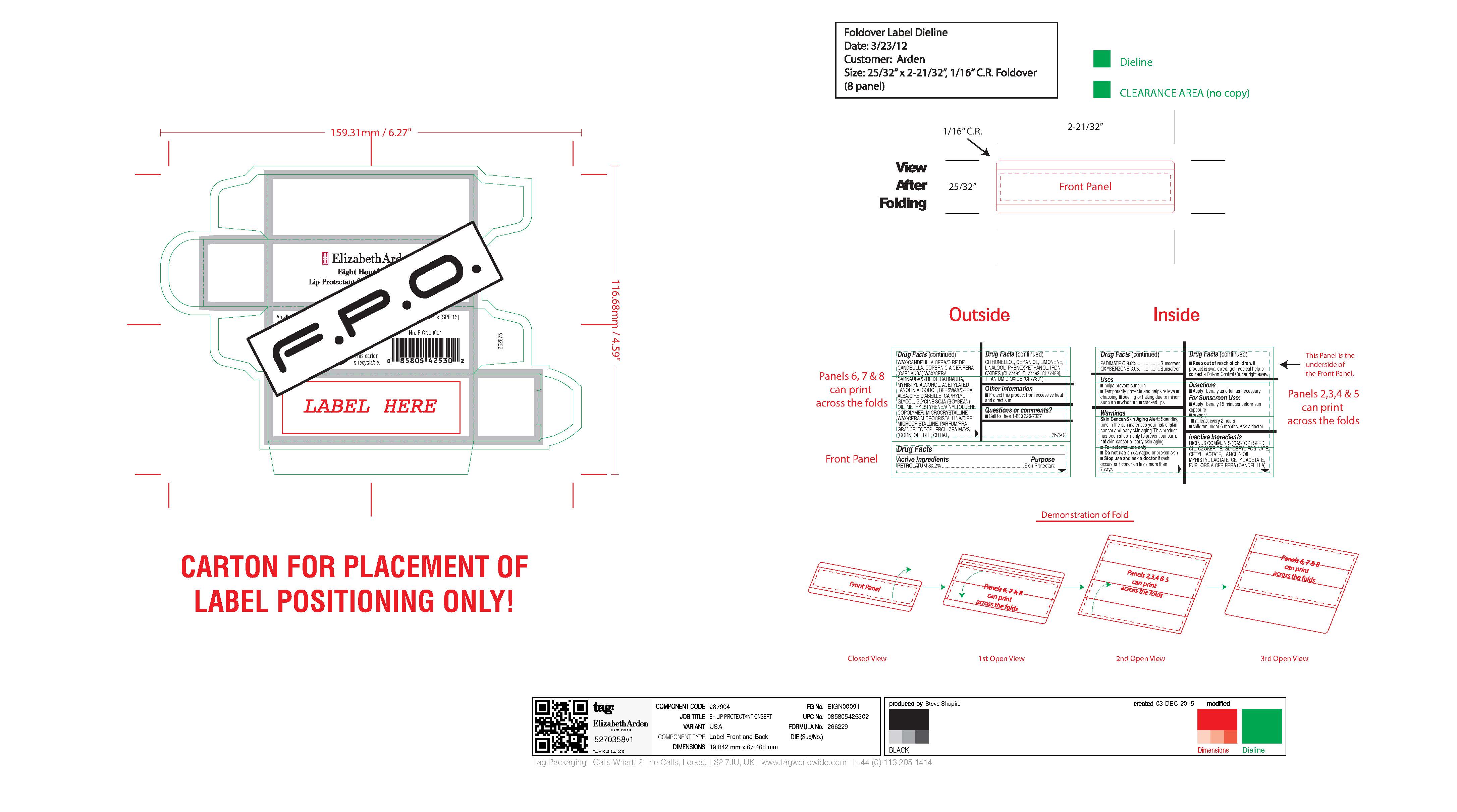
- carton art
-
INGREDIENTS AND APPEARANCE
EIGHT HOUR LIP SPF 15
petrolatum, padimate o, oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-679 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 30.2 mg in 1 g PADIMATE O (UNII: Z11006CMUZ) (PADIMATE O - UNII:Z11006CMUZ) PADIMATE O 8 mg in 1 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SOYBEAN OIL (UNII: 241ATL177A) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CORN OIL (UNII: 8470G57WFM) CITRAL (UNII: T7EU0O9VPP) GERANIOL (UNII: L837108USY) LINALOOL, (+/-)- (UNII: D81QY6I88E) CARNAUBA WAX (UNII: R12CBM0EIZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LANOLIN OIL (UNII: OVV5IIJ58F) PHENOXYETHANOL (UNII: HIE492ZZ3T) CASTOR OIL (UNII: D5340Y2I9G) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL LACTATE (UNII: 1D822OC34X) CETYL ACETATE (UNII: 4Q43814HXS) YELLOW WAX (UNII: 2ZA36H0S2V) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+)- (UNII: GFD7C86Q1W) GLYCERYL ROSINATE (UNII: SD112V492J) CETYL LACTATE (UNII: A7EVH2RK4O) MYRISTYL ALCOHOL (UNII: V42034O9PU) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CANDELILLA WAX (UNII: WL0328HX19) ACETYLATED LANOLIN ALCOHOLS (UNII: SNN716810P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-679-61 3.7 g in 1 CARTRIDGE; Type 0: Not a Combination Product 10/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/21/2020 Labeler - REVLON (788820165) Registrant - REVLON (788820165) Establishment Name Address ID/FEI Business Operations Revlon 809725570 manufacture(10967-679)