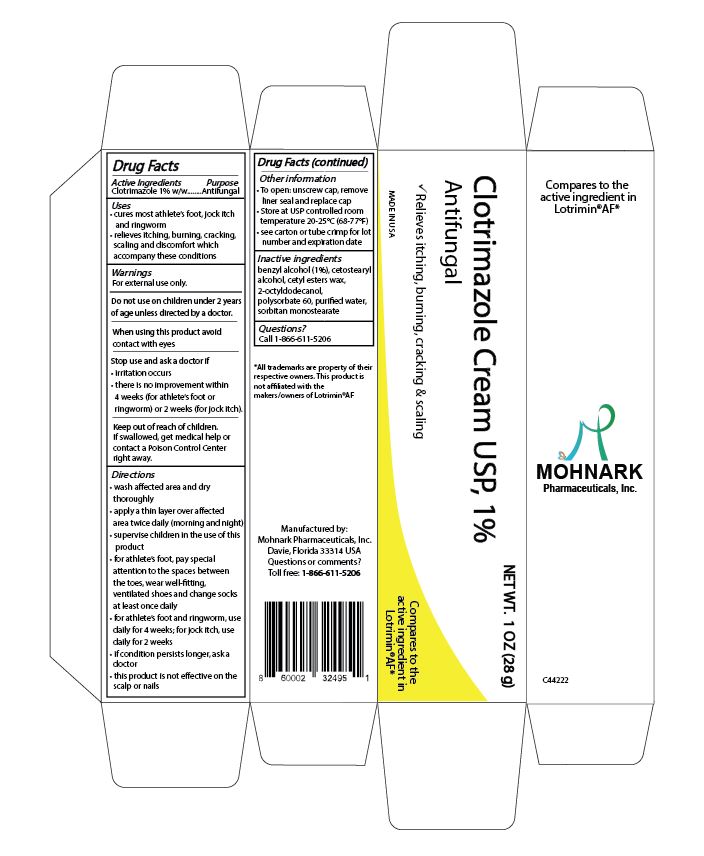
Label: CLOTRIMAZOLE 1% TOPICAL ANTIFUNGAL CREAM- clotrimazole 1% antifungal cream cream
- NDC Code(s): 73715-004-01
- Packager: Mohnark Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
For external use only
Do not use on children under 2 years of age unless directed by a doctor
When using this product avoid contact with eyes
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 4 weeks (for athlete's foot or ringworm) or 2 weeks (for jock itch)
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
- STOP USE AND ASK DOCTOR IF
-
DIRECTIONS
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot, pay special attention to the spaces between the toes, wear well fitting, ventilated shoes and change socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- DOSAGE AND ADMINISTRATION
- INDICATIONS AND USAGE
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE 1% TOPICAL ANTIFUNGAL CREAM
clotrimazole 1% antifungal cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73715-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 1 g in 100 g WATER (UNII: 059QF0KO0R) 69.5 g in 100 g POLYSORBATE 60 (UNII: CAL22UVI4M) 1.5 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73715-004-01 28 g in 1 TUBE; Type 0: Not a Combination Product 12/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/21/2020 Labeler - Mohnark Pharmaceuticals Inc. (117013830) Establishment Name Address ID/FEI Business Operations Mohnark Pharmaceuticals Inc. 117013830 manufacture(73715-004)